
The molecular weight of benzoic acid in benzene as determined by depression in the freezing point method corresponds to:
A ) ionization of benzoic acid
B ) dimerization of benzoic acid
C ) trimerization of benzoic acid
D ) solvation of benzoic acid
Answer
590.4k+ views
Hint: The molecules of benzoic acid will be associated with the help of intermolecular hydrogen bonding. Hence, the molecular weight of benzoic acid in benzene will be a whole number multiple of unassociated (monomeric) benzoic acid.
Complete answer:
The depression in the freezing point is the difference between the freezing point of a solution (containing a non volatile solvent) and the freezing point of pure solvent. The depression in the freezing point is directly proportional to the molality of the solute. This relationship is used to determine the molecular weight of the solute.
Write the formula for the depression in the freezing point:
\[\Delta {T_f} = {k_f} \times m\]
Here, \[\Delta {T_f}\] is the depression in the freezing point of solution, \[{k_f}\] is the molal depression in the freezing point constant and m is the molality of the solution.
Benzoic acid is \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}} - {\text{COOH}}\] . Its chemical formula is \[{{\text{C}}_{\text{7}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{2}}}\] . The atomic masses of carbon, hydrogen and oxygen are \[12{\text{ }}g/mol,{\text{ }}4{\text{ }}g/mol{\text{ }}and{\text{ }}16{\text{ }}g/mol\] respectively. Calculate the molecular weight of benzoic acid.
\[{\text{7}}\left( {12} \right){\text{ + 6}}\left( 1 \right){\text{ + 2}}\left( {16} \right){\text{ = 84 + 6 + 32 = 122 g/mol}}\]
When the molecular weight of the benzoic acid is determined using depression in the freezing point, it comes out to be \[244{\text{ }}g/mol\] . When 244 is divided with 122, the answer is 2.
\[\dfrac{{{\text{Molecular weight determined from depression in the freezing point method }}}}{{{\text{Molecular weight calculated from the formula }}{{\text{C}}_{\text{7}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{2}}}{\text{ }}}}{\text{ = }}\dfrac{{244}}{{122}}{\text{ = 2 }}\]
The answer 2 suggests dimerization of benzoic acid.
In benzene molecules, two molecules of benzoic acid associate to form a dimer. This dimerization is possible due to formation of intermolecular hydrogen bonds.
Hence, the option B ) is the correct option.
Additional Information The structure of the dimer of the benzoic acid in benzene is shown below:
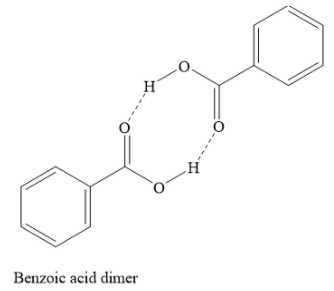
Trimerization of benzoic acid is not observed in benzene. A trimer is obtained when three molecules of benzoic acid associate. Ionization of benzoic acid will result in observed molecular weight being lower than \[122{\text{ }}g/mol\] . But this is not observed in benzene. Solvation of benzoic acid means that several benzene (solvent) molecules surround one molecule of benzoic acid. But this concept cannot explain the molecular weight of \[244{\text{ }}g/mol\] as determined by the depression in the freezing point.
Note: Intermolecular hydrogen bonds are formed between two molecules (of same or different compounds). Intermolecular hydrogen bonding is different from intramolecular hydrogen bonding. In intramolecular hydrogen bonding, hydrogen bonds are formed between two functional groups present within the same molecule.
Complete answer:
The depression in the freezing point is the difference between the freezing point of a solution (containing a non volatile solvent) and the freezing point of pure solvent. The depression in the freezing point is directly proportional to the molality of the solute. This relationship is used to determine the molecular weight of the solute.
Write the formula for the depression in the freezing point:
\[\Delta {T_f} = {k_f} \times m\]
Here, \[\Delta {T_f}\] is the depression in the freezing point of solution, \[{k_f}\] is the molal depression in the freezing point constant and m is the molality of the solution.
Benzoic acid is \[{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}} - {\text{COOH}}\] . Its chemical formula is \[{{\text{C}}_{\text{7}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{2}}}\] . The atomic masses of carbon, hydrogen and oxygen are \[12{\text{ }}g/mol,{\text{ }}4{\text{ }}g/mol{\text{ }}and{\text{ }}16{\text{ }}g/mol\] respectively. Calculate the molecular weight of benzoic acid.
\[{\text{7}}\left( {12} \right){\text{ + 6}}\left( 1 \right){\text{ + 2}}\left( {16} \right){\text{ = 84 + 6 + 32 = 122 g/mol}}\]
When the molecular weight of the benzoic acid is determined using depression in the freezing point, it comes out to be \[244{\text{ }}g/mol\] . When 244 is divided with 122, the answer is 2.
\[\dfrac{{{\text{Molecular weight determined from depression in the freezing point method }}}}{{{\text{Molecular weight calculated from the formula }}{{\text{C}}_{\text{7}}}{{\text{H}}_{\text{6}}}{{\text{O}}_{\text{2}}}{\text{ }}}}{\text{ = }}\dfrac{{244}}{{122}}{\text{ = 2 }}\]
The answer 2 suggests dimerization of benzoic acid.
In benzene molecules, two molecules of benzoic acid associate to form a dimer. This dimerization is possible due to formation of intermolecular hydrogen bonds.
Hence, the option B ) is the correct option.
Additional Information The structure of the dimer of the benzoic acid in benzene is shown below:

Trimerization of benzoic acid is not observed in benzene. A trimer is obtained when three molecules of benzoic acid associate. Ionization of benzoic acid will result in observed molecular weight being lower than \[122{\text{ }}g/mol\] . But this is not observed in benzene. Solvation of benzoic acid means that several benzene (solvent) molecules surround one molecule of benzoic acid. But this concept cannot explain the molecular weight of \[244{\text{ }}g/mol\] as determined by the depression in the freezing point.
Note: Intermolecular hydrogen bonds are formed between two molecules (of same or different compounds). Intermolecular hydrogen bonding is different from intramolecular hydrogen bonding. In intramolecular hydrogen bonding, hydrogen bonds are formed between two functional groups present within the same molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




