
The molecule having one unpaired electron is:
$
A.{\text{ }}NO \\
B.{\text{ }}CO \\
C.{\text{ }}CN \\
D.{\text{ }}{O_2} \\
$
Answer
596.4k+ views
Hint: We have to draw the electron dot structure (Lewis structures) of every given molecule and after drawing check whether they are having one unpaired electron or not.
Let’s start with the electron dot structure, as we all know every atom contains a set number of electrons in its outer shell which we called valence electrons, for example, the valence electron number for carbon is 4 whereas for chlorine it is 7. The motive of any atom is to achieve a state in which it has 8 electrons in its outer shell. To achieve such a state the atoms can share and donate the electrons. In covalent bonds, the two atoms are sharing the electrons by forming a bond between them.
Complete step by step solution:
We must know that the electron dot structure also known as Lewis structures are the diagrams that show the bonding between atoms of a molecule and also show the lone pairs of electrons which may exist in the molecule.
For drawing the Lewis dot structure the student should know the following steps:
Find the total number of valence electrons. For example, \[{O_2}\]have a total number of 6+6 =12 electrons.
If it’s a molecule with more than 2 atoms then put the least negative atom in the center. For example, in \[N{O_2}\] you will keep the nitrogen (N) between the two O i.e.\[\;O - N - O\].
Put two electrons in between the atoms to form the bond. For example, In\[HCl\], you will keep \[H:Cl\]like this a bond between H and Cl has been formed.
After forming the bond you will complete the octets on the outside atoms. For example, in case of \[HCl\]after \[H:Cl\]you will complete the octet of Cl- \[H:\underset{\raise0.3em\hbox{$\smash{\scriptscriptstyle\cdot\cdot}$}}{\ddot C} l:{\text{ }}\]like this the octet of chlorine (Cl) is complete and the Lewis dot structure is being made.
But if central atom does not have an octet then move the electrons from outside to the inside i.e. in case of \[{O_2}\]from 4th point we get $:\ddot O:\underset{\raise0.3em\hbox{$\smash{\scriptscriptstyle\cdot\cdot}$}}{\ddot O} :$this shows that one of the oxygen (O) is not having complete octet only 6 electrons so we move one electron pair from outside to the inside and we get$:\dot O::\dot O:{\text{ }}or{\text{ }}we{\text{ }}can{\text{ }}also{\text{ }}write{\text{ }}it{\text{ }}as{\text{ }}:\ddot O = \ddot O:$ like this both the atoms have a complete octet.
So let’s begin withdrawing from Lewis structures for the given molecules.
Case 1
The Lewis dot structure for \[NO\]is
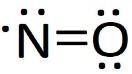
The number of the valence electrons in nitrogen (N) is 5 and in oxygen (O) is 6 which means the total electron is 11.
So, clearly \[NO\]is having one unpaired electron.
Case 2
The Lewis dot structure for\[\;CO\] is
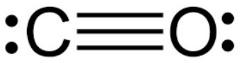
The Number of the valence electrons in Carbon (C) is 4 and in oxygen (O) is 6 which means the total electron is 10.
So clearly, \[CO\]are having all paired electrons.
Case 3
The Lewis dot structure for \[CN\]is
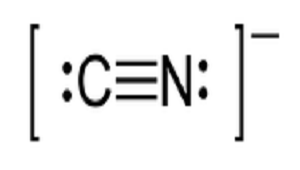
The number of the valence electron in Carbon (N) is 4 and in Nitrogen (N) is 5 which means the total electron is 9 but usually in this case a negative charge is there and the number of the electron becomes 10.
So clearly, \[CN\]is having all paired electrons.
Case 4
The Lewis dot structure for \[{O_2}\]is
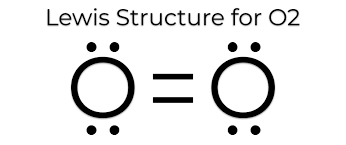
The number of the valence electron in Oxygen (O) is 6 and hence the total number of an electron is 12.
So, \[{O_2}\] is having all paired electrons.
The answer to this question is \[A.{\text{ }}NO\].
Note: We must know that In the Lewis symbol for the element in the s or p block, the number of valence electrons shall be indicated by the dots placed around the element symbol. No electron is paired until there are four single electrons in place. Electrons are coupled until eight electrons are placed. The dots are usually placed on top of the symbol, to the right, below and to the left. The order in which they are placed is unimportant.
While solving such questions the student must understand the basic concept of Lewis Structure. Also, the student must have a thorough understanding of the Valence electron of atoms which are used to form these molecules.
Let’s start with the electron dot structure, as we all know every atom contains a set number of electrons in its outer shell which we called valence electrons, for example, the valence electron number for carbon is 4 whereas for chlorine it is 7. The motive of any atom is to achieve a state in which it has 8 electrons in its outer shell. To achieve such a state the atoms can share and donate the electrons. In covalent bonds, the two atoms are sharing the electrons by forming a bond between them.
Complete step by step solution:
We must know that the electron dot structure also known as Lewis structures are the diagrams that show the bonding between atoms of a molecule and also show the lone pairs of electrons which may exist in the molecule.
For drawing the Lewis dot structure the student should know the following steps:
Find the total number of valence electrons. For example, \[{O_2}\]have a total number of 6+6 =12 electrons.
If it’s a molecule with more than 2 atoms then put the least negative atom in the center. For example, in \[N{O_2}\] you will keep the nitrogen (N) between the two O i.e.\[\;O - N - O\].
Put two electrons in between the atoms to form the bond. For example, In\[HCl\], you will keep \[H:Cl\]like this a bond between H and Cl has been formed.
After forming the bond you will complete the octets on the outside atoms. For example, in case of \[HCl\]after \[H:Cl\]you will complete the octet of Cl- \[H:\underset{\raise0.3em\hbox{$\smash{\scriptscriptstyle\cdot\cdot}$}}{\ddot C} l:{\text{ }}\]like this the octet of chlorine (Cl) is complete and the Lewis dot structure is being made.
But if central atom does not have an octet then move the electrons from outside to the inside i.e. in case of \[{O_2}\]from 4th point we get $:\ddot O:\underset{\raise0.3em\hbox{$\smash{\scriptscriptstyle\cdot\cdot}$}}{\ddot O} :$this shows that one of the oxygen (O) is not having complete octet only 6 electrons so we move one electron pair from outside to the inside and we get$:\dot O::\dot O:{\text{ }}or{\text{ }}we{\text{ }}can{\text{ }}also{\text{ }}write{\text{ }}it{\text{ }}as{\text{ }}:\ddot O = \ddot O:$ like this both the atoms have a complete octet.
So let’s begin withdrawing from Lewis structures for the given molecules.
Case 1
The Lewis dot structure for \[NO\]is
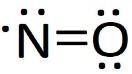
The number of the valence electrons in nitrogen (N) is 5 and in oxygen (O) is 6 which means the total electron is 11.
So, clearly \[NO\]is having one unpaired electron.
Case 2
The Lewis dot structure for\[\;CO\] is

The Number of the valence electrons in Carbon (C) is 4 and in oxygen (O) is 6 which means the total electron is 10.
So clearly, \[CO\]are having all paired electrons.
Case 3
The Lewis dot structure for \[CN\]is
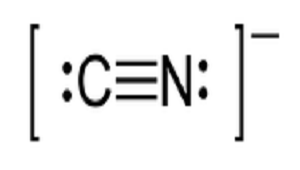
The number of the valence electron in Carbon (N) is 4 and in Nitrogen (N) is 5 which means the total electron is 9 but usually in this case a negative charge is there and the number of the electron becomes 10.
So clearly, \[CN\]is having all paired electrons.
Case 4
The Lewis dot structure for \[{O_2}\]is
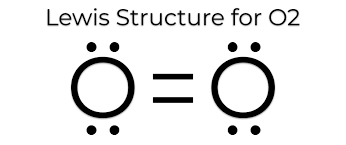
The number of the valence electron in Oxygen (O) is 6 and hence the total number of an electron is 12.
So, \[{O_2}\] is having all paired electrons.
The answer to this question is \[A.{\text{ }}NO\].
Note: We must know that In the Lewis symbol for the element in the s or p block, the number of valence electrons shall be indicated by the dots placed around the element symbol. No electron is paired until there are four single electrons in place. Electrons are coupled until eight electrons are placed. The dots are usually placed on top of the symbol, to the right, below and to the left. The order in which they are placed is unimportant.
While solving such questions the student must understand the basic concept of Lewis Structure. Also, the student must have a thorough understanding of the Valence electron of atoms which are used to form these molecules.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




