
The monomer unit for the given compound is:
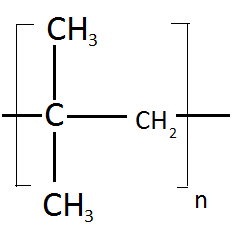
a.) 2-methylpropene
b.) Styrene
c.) Propylene
d.) Ethane

Answer
569.7k+ views
Hint: Monomer is the smallest unit or a molecule that repeats a large number of times in a polymer. We can also say it forms from the basic unit of polymers.
Complete Solution :
In the above given structure the structure of the repeating unit is as follow:
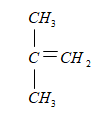
In the above structure the longest chain is containing three carbons and is also having a double bond, at the position of second carbon a methyl group is present and hence the name of the structure is
2-methylpropene
Hence the option (A) is correct.
- In option (B) that is styrene contains a benzene ring which is not present in the given polymer structure
So, the option (B) is not the correct option.
- In option (C) propylene there should be only three carbons with neither other carbons nor side chains but we are having a side chain in the given polymer structure and hence this option is also not the correct option.
- Option (D) is given as Ethane which contains only two carbons in its structure but we are having more than two carbons in the monomer structure obtained from the polymeric structure and hence this option is also not the correct option.
So, the correct answer is “Option A”.
Note: All the monomers have the capacity to form the chemical bonds to at least two other monomer molecules, and the process of formation or the connection of the bonds (which are covalent in nature) between the two monomer molecules is called dehydration synthesis.
Complete Solution :
In the above given structure the structure of the repeating unit is as follow:
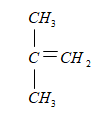
In the above structure the longest chain is containing three carbons and is also having a double bond, at the position of second carbon a methyl group is present and hence the name of the structure is
2-methylpropene
Hence the option (A) is correct.
- In option (B) that is styrene contains a benzene ring which is not present in the given polymer structure
So, the option (B) is not the correct option.
- In option (C) propylene there should be only three carbons with neither other carbons nor side chains but we are having a side chain in the given polymer structure and hence this option is also not the correct option.
- Option (D) is given as Ethane which contains only two carbons in its structure but we are having more than two carbons in the monomer structure obtained from the polymeric structure and hence this option is also not the correct option.
So, the correct answer is “Option A”.
Note: All the monomers have the capacity to form the chemical bonds to at least two other monomer molecules, and the process of formation or the connection of the bonds (which are covalent in nature) between the two monomer molecules is called dehydration synthesis.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




