
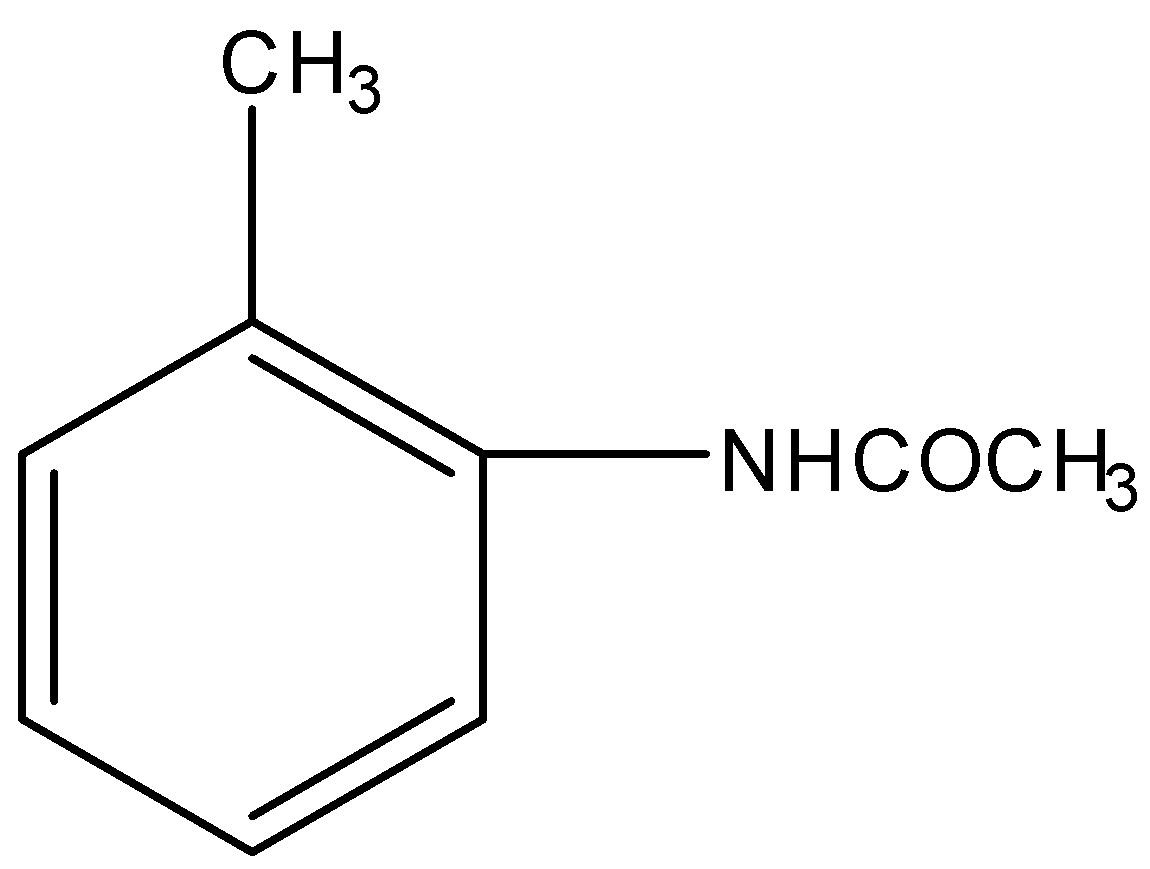
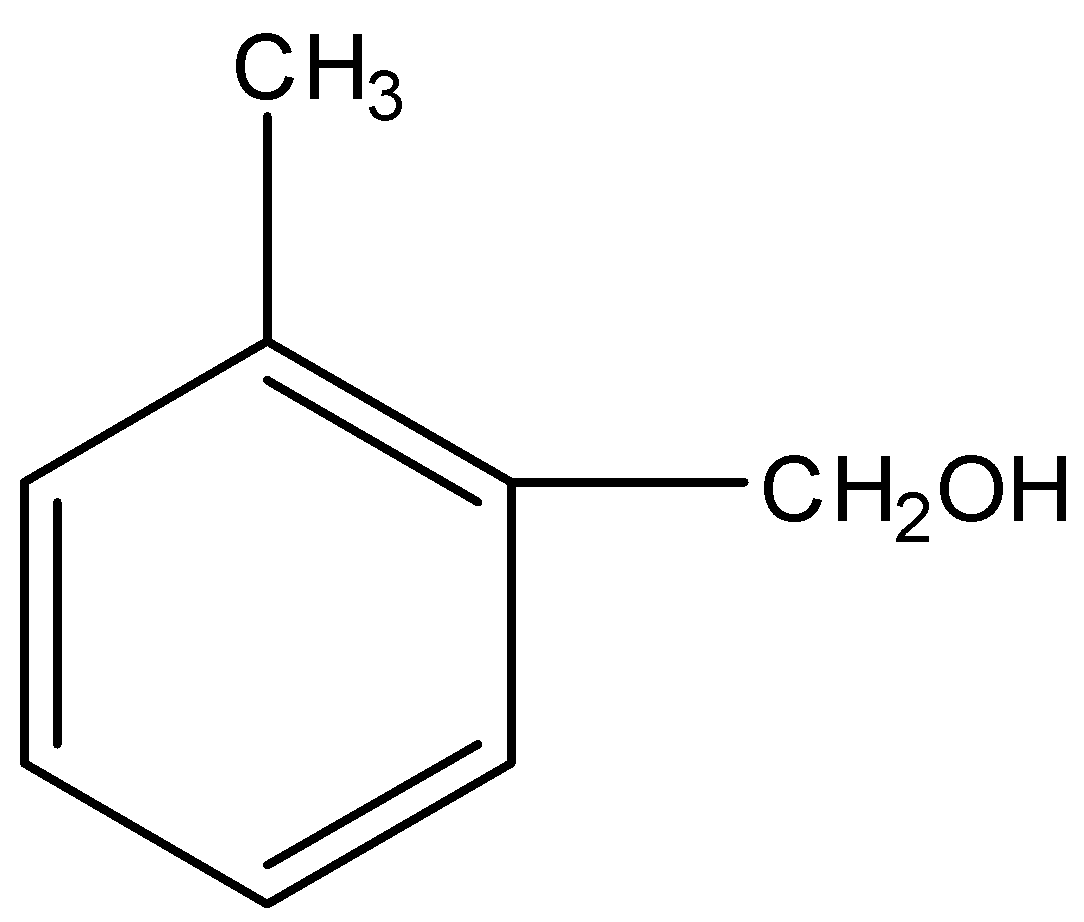
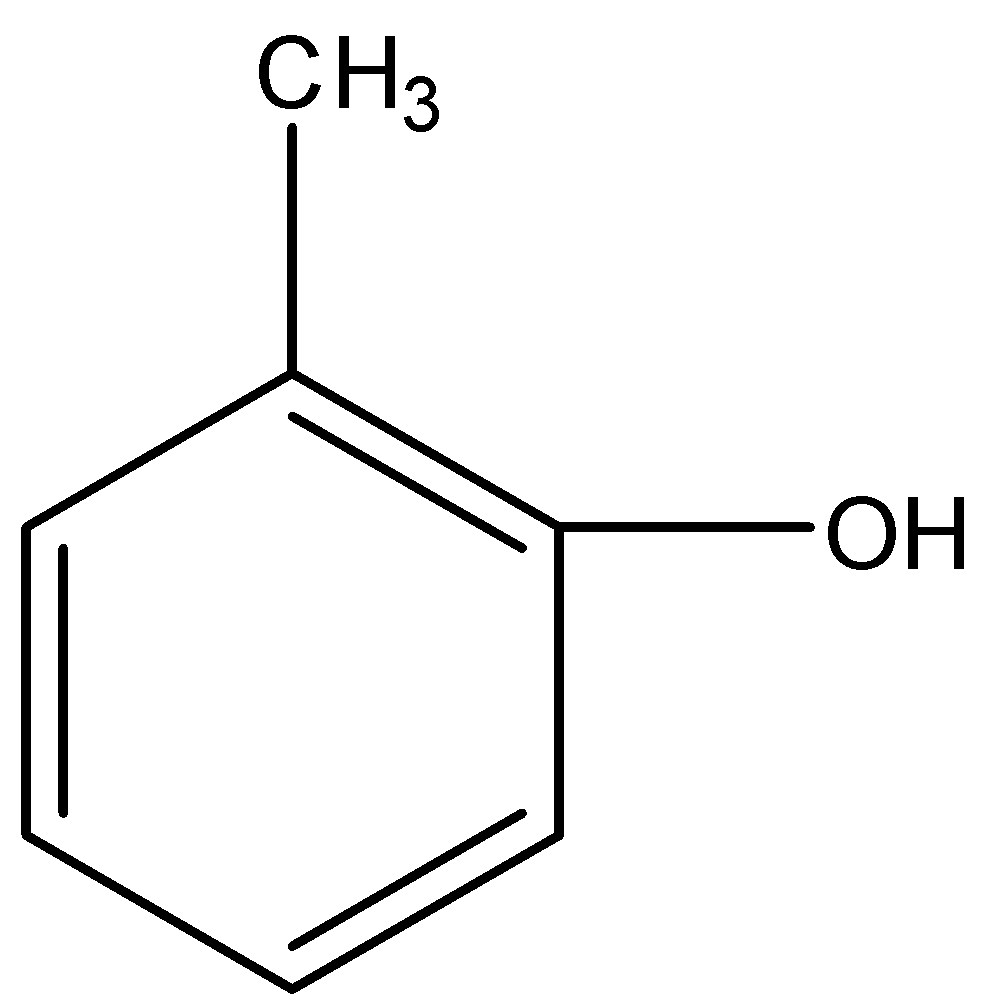
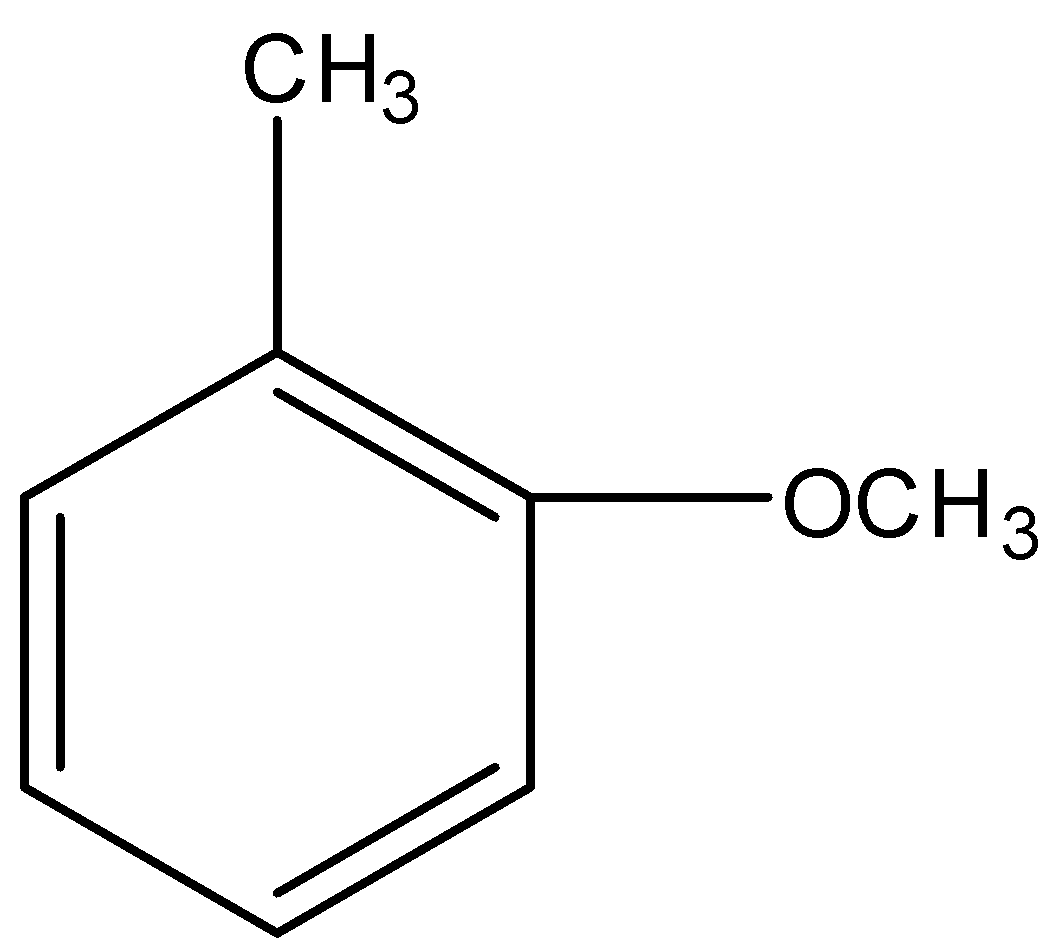
The most reactive towards electrophilic reagent is :
(A)
(B)
(C)
(D)




Answer
593.4k+ views
Hint: Electrophiles are electron deficient species. Benzene is nucleophilic (electron rich) in nature due to the presence of $\pi $ electron cloud and thus shows electrophilic substitution reactions. Electron donating groups (EDG) increase the electron density in the benzene ring and activate it towards electrophilic reagents.
Complete step by step answer:
- Reactivity of substituted benzene depends on the type of substituent present in the ring.
- Electron releasing groups increase the overall electron density and hence the reactivity of the benzene rings. Such groups mainly increase the electron density at ortho and para positions and direct the incoming group to substitute at these positions. Hence, they are called as o, p- directing groups.
- Since presence of these groups facilitates further electrophilic substitutions in the ring, they are also known as activating groups.
- Reactivity of a benzene ring substituted with o, p- directing group depends on the electron donating ability of the group attached. The electron donating ability of some EDG follows the given order:
Applying the above order in substituted toluene, we know that hydroxyl ($-OH$) group is more electron donating than $-NHCOC{{H}_{3}}$, $-OC{{H}_{3}}$ and $-C{{H}_{2}}OH$.
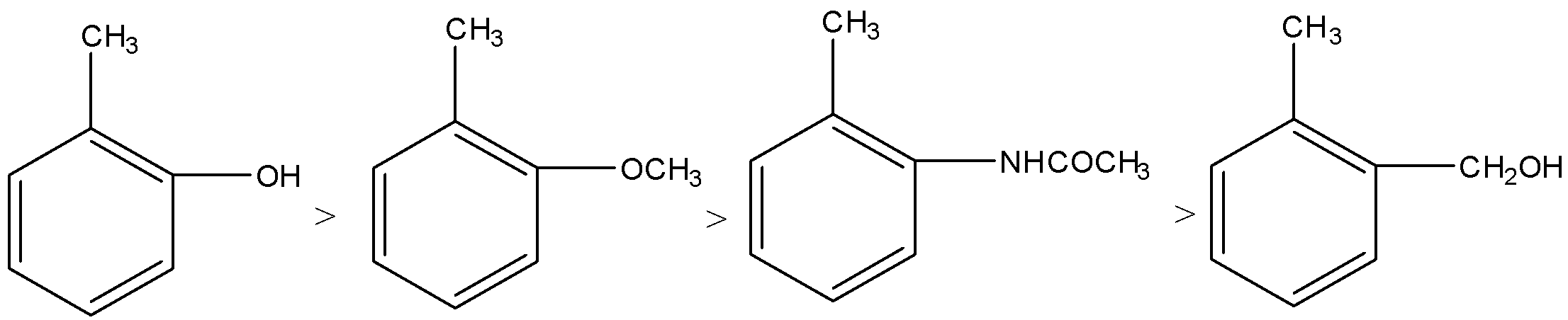
Therefore, o-hydroxy toluene is most reactive towards electrophilic reagent.
So, the correct answer is “Option D”.
Additional Information:
Electron Withdrawing groups (EWG) withdraw electron density from the ring (particularly from the meta-position) and deactivate the ring toward electrophones. They are also called as m-directing groups. $-{{N}^{\oplus }}{{R}_{3}}$, $-N{{O}_{2}}$, -CN, etc are strong EWG.
Note: Although EDG equally directs the incoming groups at o and p- positions. However, if the incoming group or the EDG already present on the ring is bulky, the incoming group generally attaches at the p-position to avoid steric hindrance with EDG which may arise if it is attached at the o-position.
Complete step by step answer:
- Reactivity of substituted benzene depends on the type of substituent present in the ring.
- Electron releasing groups increase the overall electron density and hence the reactivity of the benzene rings. Such groups mainly increase the electron density at ortho and para positions and direct the incoming group to substitute at these positions. Hence, they are called as o, p- directing groups.
- Since presence of these groups facilitates further electrophilic substitutions in the ring, they are also known as activating groups.
- Reactivity of a benzene ring substituted with o, p- directing group depends on the electron donating ability of the group attached. The electron donating ability of some EDG follows the given order:
\[-N{{H}_{2}}>-N{{R}_{2}}>-OH>-OR>-NHCOR>-R\]
Applying the above order in substituted toluene, we know that hydroxyl ($-OH$) group is more electron donating than $-NHCOC{{H}_{3}}$, $-OC{{H}_{3}}$ and $-C{{H}_{2}}OH$.

Therefore, o-hydroxy toluene is most reactive towards electrophilic reagent.
So, the correct answer is “Option D”.
Additional Information:
Electron Withdrawing groups (EWG) withdraw electron density from the ring (particularly from the meta-position) and deactivate the ring toward electrophones. They are also called as m-directing groups. $-{{N}^{\oplus }}{{R}_{3}}$, $-N{{O}_{2}}$, -CN, etc are strong EWG.
Note: Although EDG equally directs the incoming groups at o and p- positions. However, if the incoming group or the EDG already present on the ring is bulky, the incoming group generally attaches at the p-position to avoid steric hindrance with EDG which may arise if it is attached at the o-position.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




