
The name of the compound $C{{H}_{3}}-C{{H}_{2}}-CHO$ is:
A. Propanal
B. Propanone
C. Ethanol
D. Ethanal
Answer
592.8k+ views
Hint: In order to know the name of the compound we should be familiar with the rules of nomenclature. We should also have an idea about the structure of the compound. Think about what prefix is added according to the parent alkyl group and the suffix added for the aldehyde functional group.
Complete answer:
The very first step, of naming a compound, is identifying the functional group in the compound. In this case it is aldehyde ($-CHO$). The total number of carbon atoms present in the compound is 3.
So the name of the compound will be having a prefix of prop (due to the presence of 3 carbon atoms). And as per the functional group, it will be ‘al’.
Therefore, the name of the compound is propanal.
The structure of the propanal is given below:
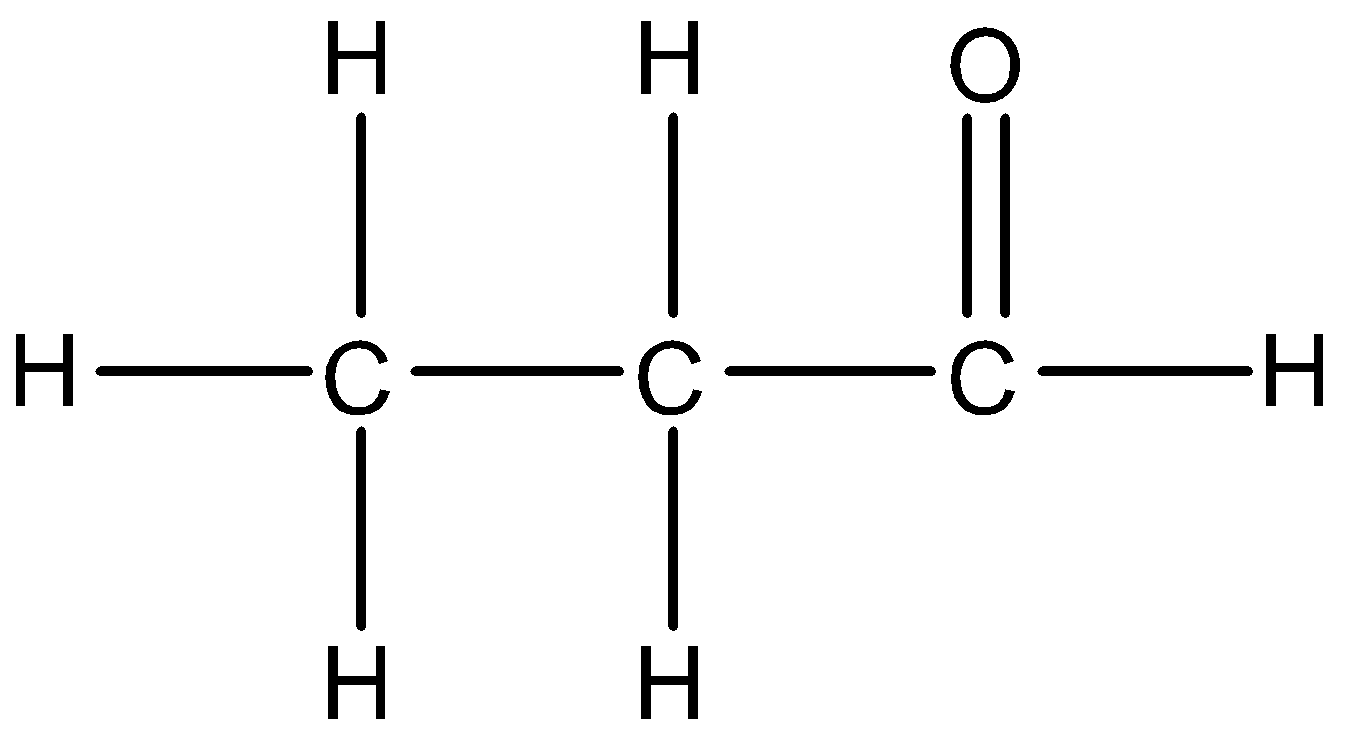
Now, moving on to the second option we have Propanone. There are 3 carbon atoms and the functional group is ketone.
The structure of propanone will be:
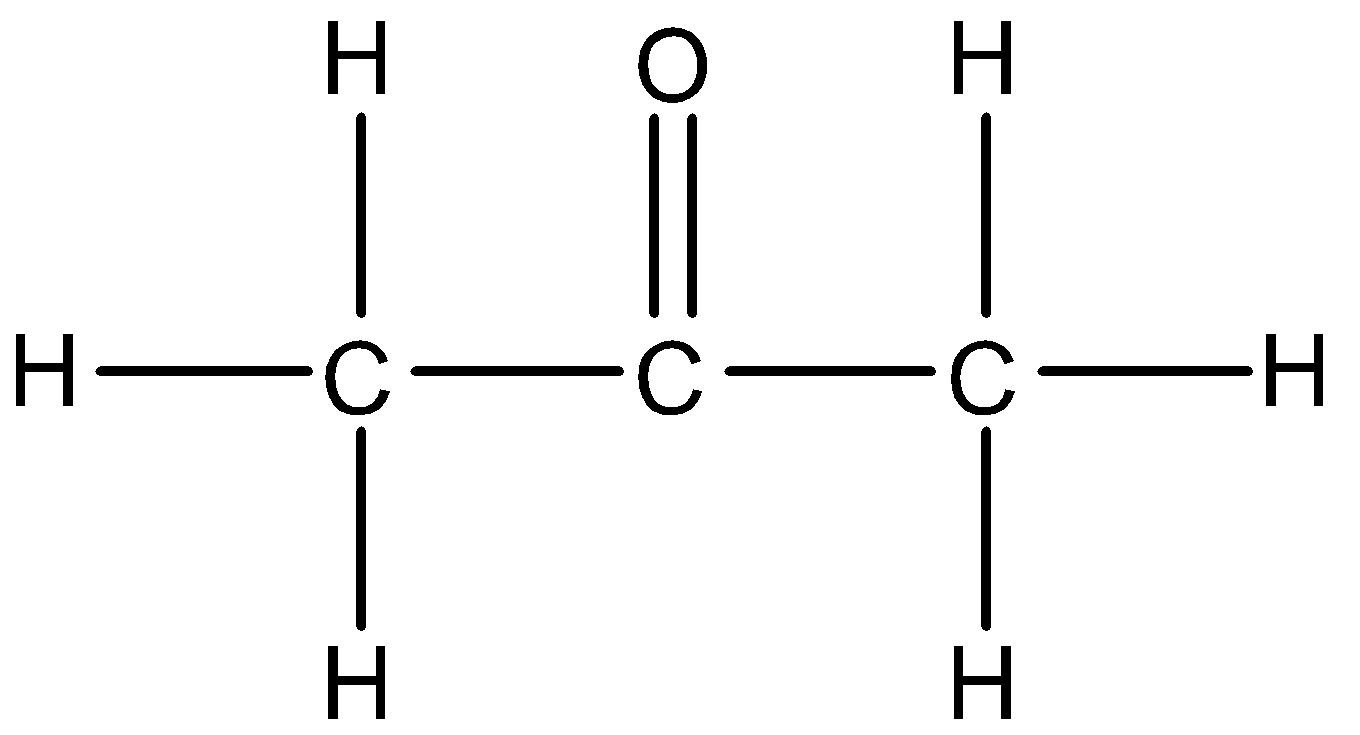
This does not resemble the compound mentioned in the question, since there is the presence of a different functional group and also a double bond. So, Option B is not the answer.
The third option is Ethanol. By ‘eth’ we mean 2 carbon atoms. But the compound in the question has 3 carbon atoms, so this does not resemble the answer.
The structure of ethanol:
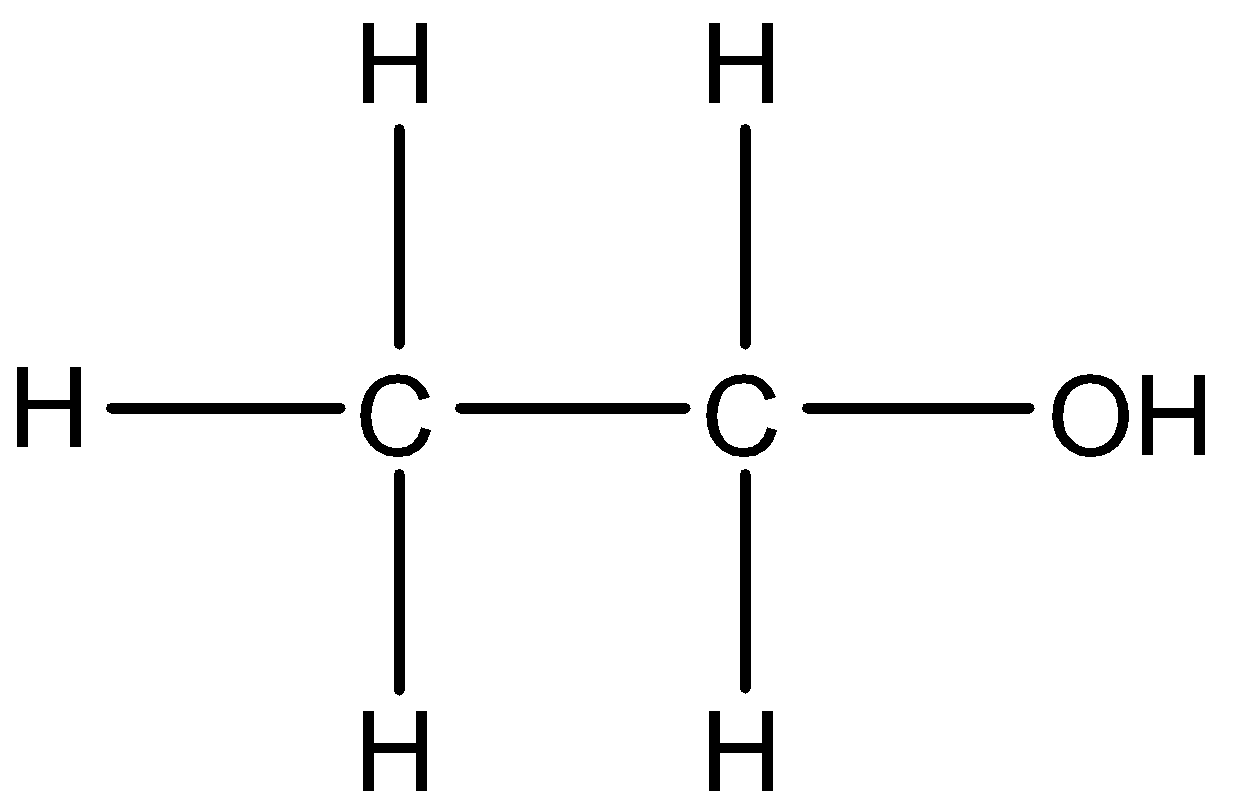
The last option is ethanal. The name eth signifies 2 carbon atoms, so it cannot be the answer. Moreover, it has got a double bond.
The structure of ethanal is given below:
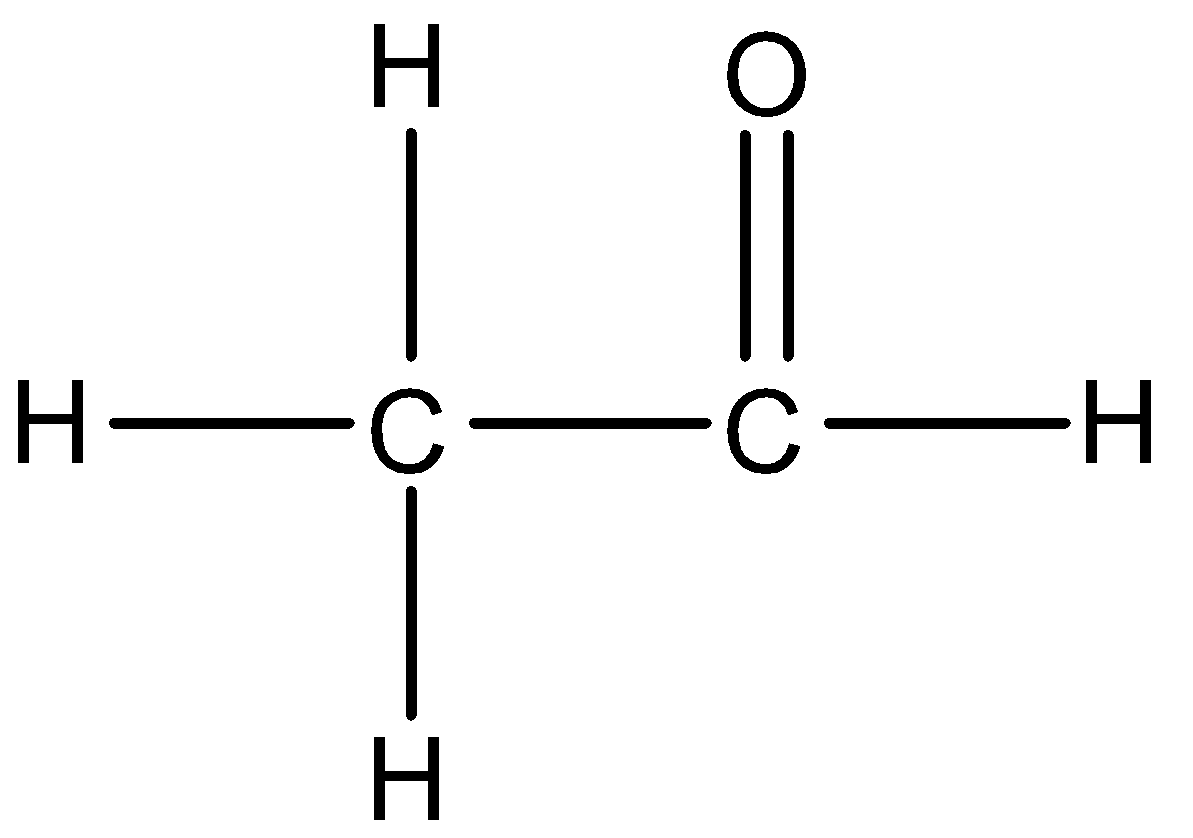
So, the correct answer is “Option A”.
Note: Remember that the prefix prop- comes from the total number of carbon atoms present in the molecule and not the number of carbon atoms present in the alkyl group attached to the carbon atom. We can also write the formula of propanal as ${{C}_{3}}{{H}_{6}}O$.
Complete answer:
The very first step, of naming a compound, is identifying the functional group in the compound. In this case it is aldehyde ($-CHO$). The total number of carbon atoms present in the compound is 3.
So the name of the compound will be having a prefix of prop (due to the presence of 3 carbon atoms). And as per the functional group, it will be ‘al’.
Therefore, the name of the compound is propanal.
The structure of the propanal is given below:

Now, moving on to the second option we have Propanone. There are 3 carbon atoms and the functional group is ketone.
The structure of propanone will be:

This does not resemble the compound mentioned in the question, since there is the presence of a different functional group and also a double bond. So, Option B is not the answer.
The third option is Ethanol. By ‘eth’ we mean 2 carbon atoms. But the compound in the question has 3 carbon atoms, so this does not resemble the answer.
The structure of ethanol:

The last option is ethanal. The name eth signifies 2 carbon atoms, so it cannot be the answer. Moreover, it has got a double bond.
The structure of ethanal is given below:

So, the correct answer is “Option A”.
Note: Remember that the prefix prop- comes from the total number of carbon atoms present in the molecule and not the number of carbon atoms present in the alkyl group attached to the carbon atom. We can also write the formula of propanal as ${{C}_{3}}{{H}_{6}}O$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




