
The name of the following compound is isobutyl alcohol.
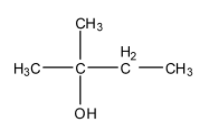
A. True
B. False

Answer
577.5k+ views
Hint: Think about the rules of nomenclature that are considered when the common name for any compound is decided. Take into consideration the number of carbon atoms that are present in the molecule and how the common name is given according to that.
Complete answer:
First, let us look at the number of carbon atoms that are present in the given molecule and how it can be related with the name that is given in the question. We can see that the structure given in the question contains 5 carbon atoms. The name in the alkyl groups series for five carbon atoms is pentane or some derivative of the suffix ‘pent-’. Unlike the IUPAC nomenclature of any molecule, we do not consider the substituents and the parent chain separately while giving the common names of small molecules. So, the common name should include the suffix ‘pent-’ and not ‘but-’ which is usually used when only 4 carbon atoms are present.
Furthermore, in an iso- alcohol, the alcoholic functional group has to be attached to any of the primary carbon atoms that are present in the iso-group and not the tertiary carbon atom, as is given in the diagram of this question. This makes the alcohol a neo- alcohol.
Hence, the answer to this question is ‘B. False’.
Note:
The actual structure of isobutyl alcohol will be:
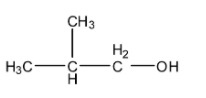
The name of the molecule whose structure is given in the question will be 2-methylbutan-2-ol. It is quite difficult to determine the common name for this molecule.
Complete answer:
First, let us look at the number of carbon atoms that are present in the given molecule and how it can be related with the name that is given in the question. We can see that the structure given in the question contains 5 carbon atoms. The name in the alkyl groups series for five carbon atoms is pentane or some derivative of the suffix ‘pent-’. Unlike the IUPAC nomenclature of any molecule, we do not consider the substituents and the parent chain separately while giving the common names of small molecules. So, the common name should include the suffix ‘pent-’ and not ‘but-’ which is usually used when only 4 carbon atoms are present.
Furthermore, in an iso- alcohol, the alcoholic functional group has to be attached to any of the primary carbon atoms that are present in the iso-group and not the tertiary carbon atom, as is given in the diagram of this question. This makes the alcohol a neo- alcohol.
Hence, the answer to this question is ‘B. False’.
Note:
The actual structure of isobutyl alcohol will be:

The name of the molecule whose structure is given in the question will be 2-methylbutan-2-ol. It is quite difficult to determine the common name for this molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




