
The nucleus of atom consists of :
(A) Protons and neutrons
(B) Protons and electrons
(C) Neutrons and electrons
(D) Protons, neutrons and electrons.
Answer
594k+ views
Hint: Nucleus of an atom is positively charged. Protons are positively charged, electrons are negatively charged whereas neutrons are chargeless species. Most of the mass is due to the presence of these two subatomic particles.
Complete answer:
Considering the charge, atoms are neutral.
Atom is made up of particles such as protons, electrons and neutrons.
As we know that the opposite charges attract each other. In the same way, the positively charged nucleus holds the negatively charged electrons in an atom.
Since, the nucleus is positively charged, it is made up of protons and neutrons.
A diagrammatic representation of atom is given below:
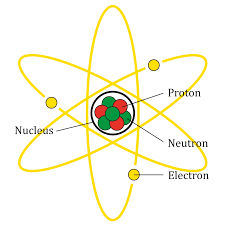
We will now go through the options to find the correct option.
Option (A) protons and neutrons is the correct answer. Since the protons are positively charged and neutrons are chargeless species, they together combine to form a positively charged nucleus.
Option (B) Protons and electrons is a wrong option. Since, protons are positively charged and electrons are negatively charged and they together when combined will result in a neutral nucleus, which is contradictory to the fact that nucleus is positively charged.
Option (C) Neutrons and electrons is a wrong option. Since, neutrons are chargeless species and electrons are negatively charged and they together when combined will result in a negatively charged nucleus, which is contradictory to the fact that nucleus is positively charged.
Option (D) Protons, neutrons and electrons is a wrong option, which contradicts the fact that the nucleus holds the electrons in an atom( by electrostatic force) and for that to happen electrons cannot be inside the nucleus.
The correct answer is Option (A) Protons and Neutrons.
So, the correct answer is “Option A”.
Note: Nucleus is a part of the atom, where the protons and neutrons reside. Electrons continuously revolve around the nucleus. In general, we can say that the structure of an atom consists of electrons and nucleus.
Complete answer:
Considering the charge, atoms are neutral.
Atom is made up of particles such as protons, electrons and neutrons.
As we know that the opposite charges attract each other. In the same way, the positively charged nucleus holds the negatively charged electrons in an atom.
Since, the nucleus is positively charged, it is made up of protons and neutrons.
A diagrammatic representation of atom is given below:

We will now go through the options to find the correct option.
Option (A) protons and neutrons is the correct answer. Since the protons are positively charged and neutrons are chargeless species, they together combine to form a positively charged nucleus.
Option (B) Protons and electrons is a wrong option. Since, protons are positively charged and electrons are negatively charged and they together when combined will result in a neutral nucleus, which is contradictory to the fact that nucleus is positively charged.
Option (C) Neutrons and electrons is a wrong option. Since, neutrons are chargeless species and electrons are negatively charged and they together when combined will result in a negatively charged nucleus, which is contradictory to the fact that nucleus is positively charged.
Option (D) Protons, neutrons and electrons is a wrong option, which contradicts the fact that the nucleus holds the electrons in an atom( by electrostatic force) and for that to happen electrons cannot be inside the nucleus.
The correct answer is Option (A) Protons and Neutrons.
So, the correct answer is “Option A”.
Note: Nucleus is a part of the atom, where the protons and neutrons reside. Electrons continuously revolve around the nucleus. In general, we can say that the structure of an atom consists of electrons and nucleus.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




