
The number of chiral carbon in glucose is
Answer
507k+ views
Hint: We need to know that they amend the idea of chirality. Discover the significance and models of appointing a chiral carbon or an unbalanced carbon molecule. Attract the construction of glucose, fischer projection recipe and afterward mark the chiral carbons present in glucose. Check all the chiral carbons and find the solution.
Complete answer:
We have to know that chirality is the property of deviation in an atom or any substance. In the event that the perfect representation of a substance is non-superimposable with that substance then, at that point, that substance is said to have chirality and the substance is supposed to be chiral or awry. It is addressed by an asterisk $\left( * \right)$ .
For instance, the letter A has the perfect representation A in particular. So both the pictures can be superimposed as one picture. So, the letter A isn't chiral or achiral.
For letter B or D or P its perfect representations are non-superimposable to one another and in this way, these letters are chiral and enantiomers of one another.
The structure of glucose is given below,
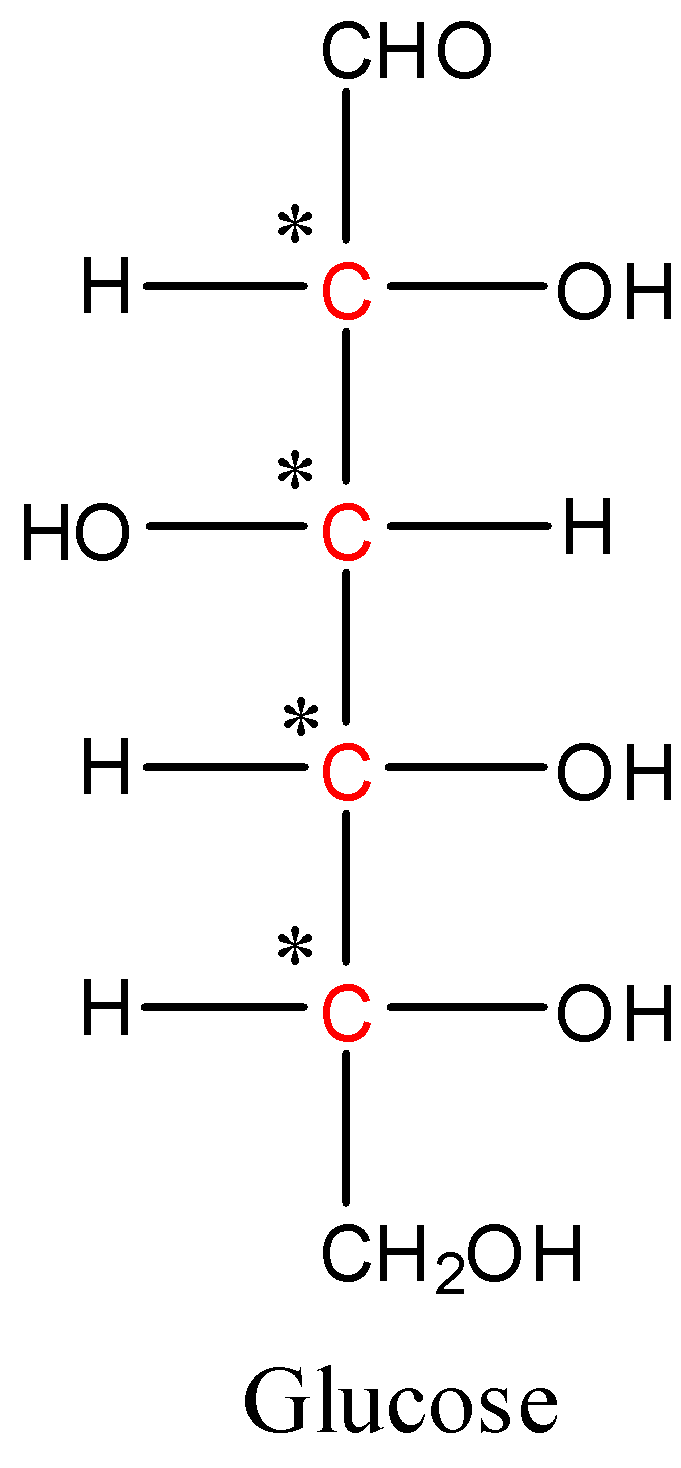
Glucose has six carbon atoms. Out of that, first and last carbon are unquestionably achiral in light of the fact that one carbon is unsaturated and another is framing two securities with hydrogen molecules. The center four carbon molecules in the chain are chiral on the grounds that they all unmistakably have four diverse substituents appended to them. They are displayed in red ink above. In this manner, the quantity of chiral carbon particles in glucose are four.
Note:
We have to see that, the other model is of hand, take a gander at the palms of both of your hands. They are perfect representations of one another. However, when you keep your right hand palm on your left hand palm, such as stacking, they don't superimpose on one another.
Complete answer:
We have to know that chirality is the property of deviation in an atom or any substance. In the event that the perfect representation of a substance is non-superimposable with that substance then, at that point, that substance is said to have chirality and the substance is supposed to be chiral or awry. It is addressed by an asterisk $\left( * \right)$ .
For instance, the letter A has the perfect representation A in particular. So both the pictures can be superimposed as one picture. So, the letter A isn't chiral or achiral.
For letter B or D or P its perfect representations are non-superimposable to one another and in this way, these letters are chiral and enantiomers of one another.
The structure of glucose is given below,

Glucose has six carbon atoms. Out of that, first and last carbon are unquestionably achiral in light of the fact that one carbon is unsaturated and another is framing two securities with hydrogen molecules. The center four carbon molecules in the chain are chiral on the grounds that they all unmistakably have four diverse substituents appended to them. They are displayed in red ink above. In this manner, the quantity of chiral carbon particles in glucose are four.
Note:
We have to see that, the other model is of hand, take a gander at the palms of both of your hands. They are perfect representations of one another. However, when you keep your right hand palm on your left hand palm, such as stacking, they don't superimpose on one another.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




