
The number of chiral centers in open-chain structures of glucose is
A.3
B.4
C.5
D.6
Answer
576k+ views
Hint: We know that the chiral center is a point at which all the four groups surrounding the point are of different atoms. An example of it is $C(F)(Cl)(Br)H$. The molecular formula of Glucose is
${{{C}}_{{6}}}{{{H}}_{{{12}}}}{{{O}}_{{6}}}$. From the open chain structure of glucose, the number of chiral centers present in the glucose molecule can be found out.
Complete step by step answer:
As we know that here, the chiral center is a carbon that is attached to four different groups of atoms.
The open-chain structure of glucose is drawn as -
.
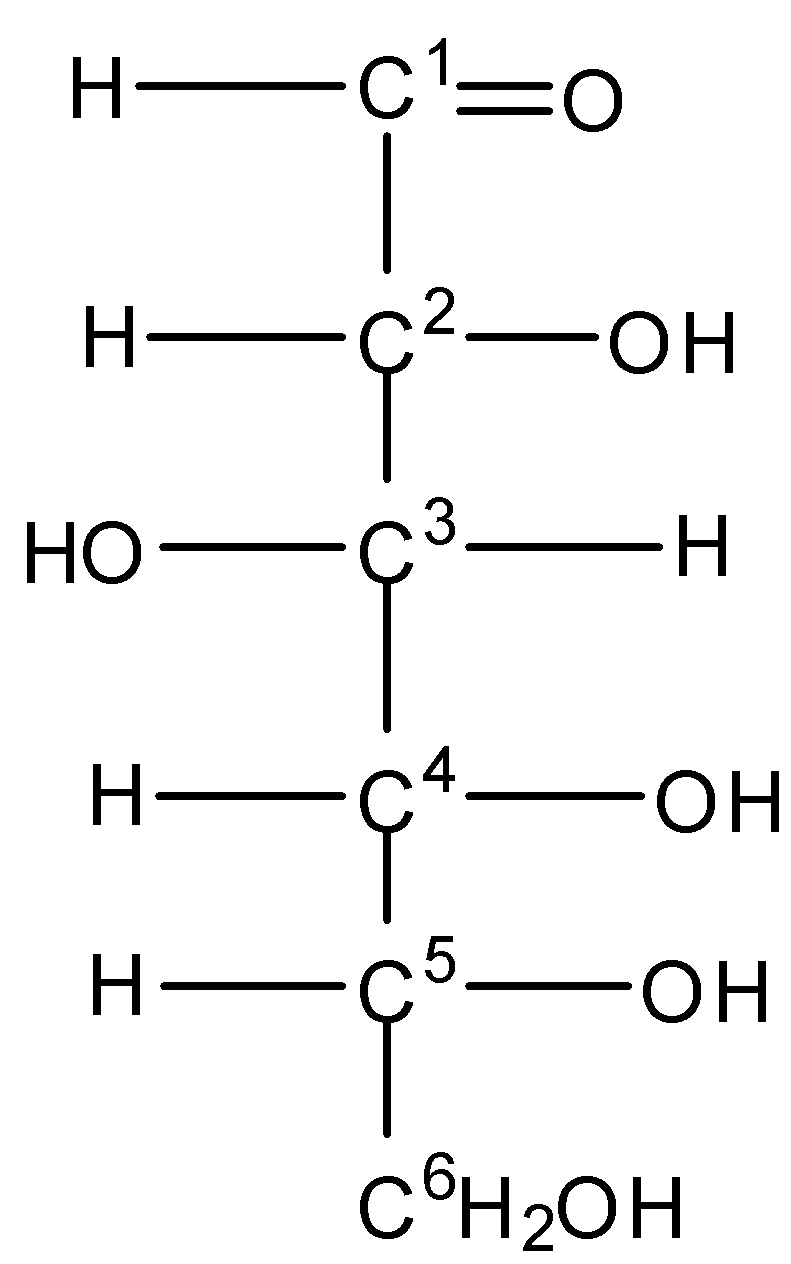
Here we can see that there is no internal plane of symmetry.
In ${C^1}$, there is a double bond to Oxygen, so we can consider it a 2 oxygen atom. So it is not a chiral carbon.
On checking the ${C^2},{C^3},{C^4},{C^5}$ , the valency of carbon is satisfied by 4 different groups of atoms. Hence they are chiral.
Therefore the total number of chiral centers present in the open-chain structure of glucose is four.
Therefore, the atomic masses of the element X and Y are 25.6 and 42.6
So, the correct answer is Option B.
Note: The open-chain structure of glucose has 4 chiral centers. The cyclic structure of glucose resembles pyran and due to the formation of cyclic hemiacetal, there are five chiral centers present in the cyclic form of glucose.
${{{C}}_{{6}}}{{{H}}_{{{12}}}}{{{O}}_{{6}}}$. From the open chain structure of glucose, the number of chiral centers present in the glucose molecule can be found out.
Complete step by step answer:
As we know that here, the chiral center is a carbon that is attached to four different groups of atoms.
The open-chain structure of glucose is drawn as -
.

Here we can see that there is no internal plane of symmetry.
In ${C^1}$, there is a double bond to Oxygen, so we can consider it a 2 oxygen atom. So it is not a chiral carbon.
On checking the ${C^2},{C^3},{C^4},{C^5}$ , the valency of carbon is satisfied by 4 different groups of atoms. Hence they are chiral.
Therefore the total number of chiral centers present in the open-chain structure of glucose is four.
Therefore, the atomic masses of the element X and Y are 25.6 and 42.6
So, the correct answer is Option B.
Note: The open-chain structure of glucose has 4 chiral centers. The cyclic structure of glucose resembles pyran and due to the formation of cyclic hemiacetal, there are five chiral centers present in the cyclic form of glucose.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




