
The number of meso diastereomers of ${C_6}{H_5}C{l_2}$ is-
A.$2$
B.$3$
C.$4$
D.$5$
Answer
582.3k+ views
Hint: Meso compounds are compounds containing chiral centers and have plane of symmetry which means they can be divided into two halves which are mirror images of each other. Due to this plane of symmetry they are generally optically inactive.
Complete step by step answer:
The properties of meso compounds are given as-
Meso compounds are such compounds which have a chiral center means the carbon is attached with different atoms to fulfill its tetra-valency.
These compounds have a plane of symmetry present. This means that when we bisect the molecule it forms two equal halves that are mirror images of each other.
They are optically inactive as they are super imposable which means it cannot rotate plane polarized light.
The compound is ${C_6}{H_5}C{l_2}$. It will have two meso diastereomers which are given as-
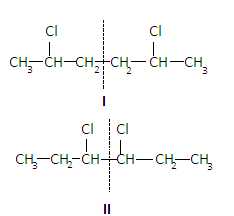
The line dividing the structure I and II shows a plane of symmetry in the molecules.
Hence the correct answer is A.
Note:
-The compound given in the question is called hydrophenylchlorin. Sometimes meso compounds are called achiral diastereomers. Let us make this clear that it does not mean that the meso compound will not have chiral carbons.
-A meso compound has a chiral center but it also has an internal plane of symmetry.
-This makes the molecule achiral. Hence we can call them achiral diastereomers.
-Also it does not rotate plane polarized light as it is super imposable. So it does not have enantiomers.
-Enantiomers are compounds that are non-superimposable mirror images of each other.
Complete step by step answer:
The properties of meso compounds are given as-
Meso compounds are such compounds which have a chiral center means the carbon is attached with different atoms to fulfill its tetra-valency.
These compounds have a plane of symmetry present. This means that when we bisect the molecule it forms two equal halves that are mirror images of each other.
They are optically inactive as they are super imposable which means it cannot rotate plane polarized light.
The compound is ${C_6}{H_5}C{l_2}$. It will have two meso diastereomers which are given as-
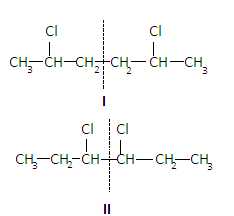
The line dividing the structure I and II shows a plane of symmetry in the molecules.
Hence the correct answer is A.
Note:
-The compound given in the question is called hydrophenylchlorin. Sometimes meso compounds are called achiral diastereomers. Let us make this clear that it does not mean that the meso compound will not have chiral carbons.
-A meso compound has a chiral center but it also has an internal plane of symmetry.
-This makes the molecule achiral. Hence we can call them achiral diastereomers.
-Also it does not rotate plane polarized light as it is super imposable. So it does not have enantiomers.
-Enantiomers are compounds that are non-superimposable mirror images of each other.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




