
The number of \[\pi \] electrons present in naphthalene is-
a.6
b.10
c.5
d.12
Answer
492.9k+ views
Hint: The chemical compound naphthalene has the formula \[{C_{10}}{H_8}\]. It's the simplest polycyclic aromatic hydrocarbon, and it's a white crystalline solid with a distinct odour perceptible at concentrations as low as 0.08 parts per million by mass. The structure of naphthalene as an aromatic hydrocarbon is made up of a fused pair of benzene rings. It's well recognised for being the primary component in mothballs.
Complete answer:
Pi bonds are covalent chemical connections in chemistry that arise when two lobes of an orbital on one atom overlap two lobes of an orbital on another atom laterally. At a common nodal plane running across the two bound nuclei, each of these atomic orbitals has zero electron density. The pi bond molecular orbital has a nodal plane in the same plane. Pi bonds can occur in double and triple bonds, but they almost never form in single bonds. Because the orbital symmetry of the pi bond is the same as that of the p orbital when viewed down the bond axis, the Greek letter\[\pi \] in their name alludes to p orbitals.
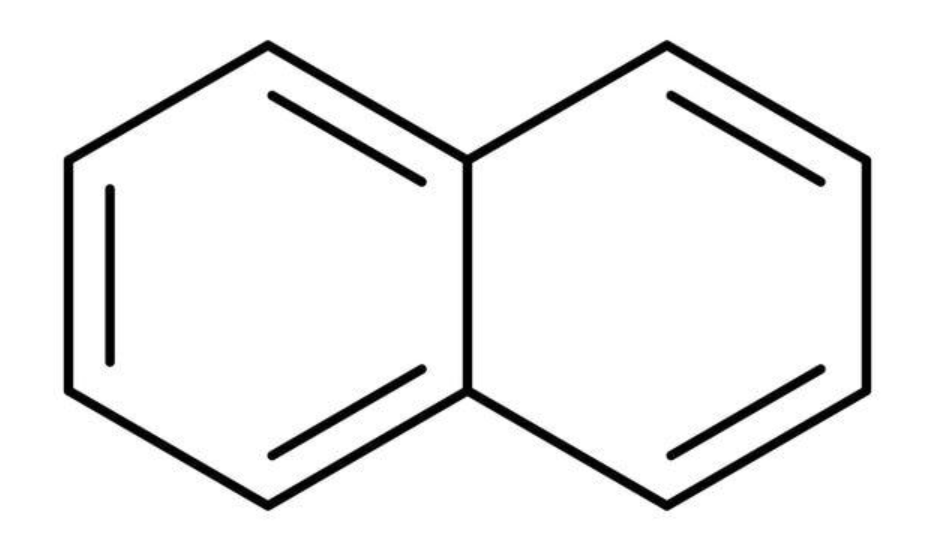
Total \[\pi \] bonds = 5
Total number of \[\pi \] electrons = 10
Note:
Pi bonds are formed when two regions of overlap of atomic orbitals come into contact. The sigma bonds are more diffuse than the pi bonds. Pi electrons are electrons in pi bonds that are occasionally referred to as such. Because rotation destroys the parallel alignment of the constituent p orbitals, molecular fragments linked by a pi bond cannot rotate around that connection without breaking the pi bond.
Complete answer:
Pi bonds are covalent chemical connections in chemistry that arise when two lobes of an orbital on one atom overlap two lobes of an orbital on another atom laterally. At a common nodal plane running across the two bound nuclei, each of these atomic orbitals has zero electron density. The pi bond molecular orbital has a nodal plane in the same plane. Pi bonds can occur in double and triple bonds, but they almost never form in single bonds. Because the orbital symmetry of the pi bond is the same as that of the p orbital when viewed down the bond axis, the Greek letter\[\pi \] in their name alludes to p orbitals.

Total \[\pi \] bonds = 5
Total number of \[\pi \] electrons = 10
Note:
Pi bonds are formed when two regions of overlap of atomic orbitals come into contact. The sigma bonds are more diffuse than the pi bonds. Pi electrons are electrons in pi bonds that are occasionally referred to as such. Because rotation destroys the parallel alignment of the constituent p orbitals, molecular fragments linked by a pi bond cannot rotate around that connection without breaking the pi bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




