
The number of $p\pi -d\pi $ bonds present in monomeric $S{{O}_{3}}$ is:
Answer
584.4k+ views
Hint: The interaction of p and d orbitals of different atoms in the formation of pi bond is called $p\pi -d\pi $ bond. To know the number of $p\pi -d\pi $ bonds we should know the electronic configuration of the atoms participating in the bond formation.
Complete step by step answer:
- The given molecule is sulphur trioxide ( $S{{O}_{3}}$ ).
- The ground state electronic configuration of the sulphur is as follows.
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{4}}\]
- The excited state electronic configuration of the sulphur is as follows.
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{1}}3{{p}^{3}}3{{d}^{2}}\]
- The electronic configuration of oxygen is as follows.
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}\]
- The overlapping of orbitals between oxygen and sulphur atoms in sulphur trioxide is as follows.
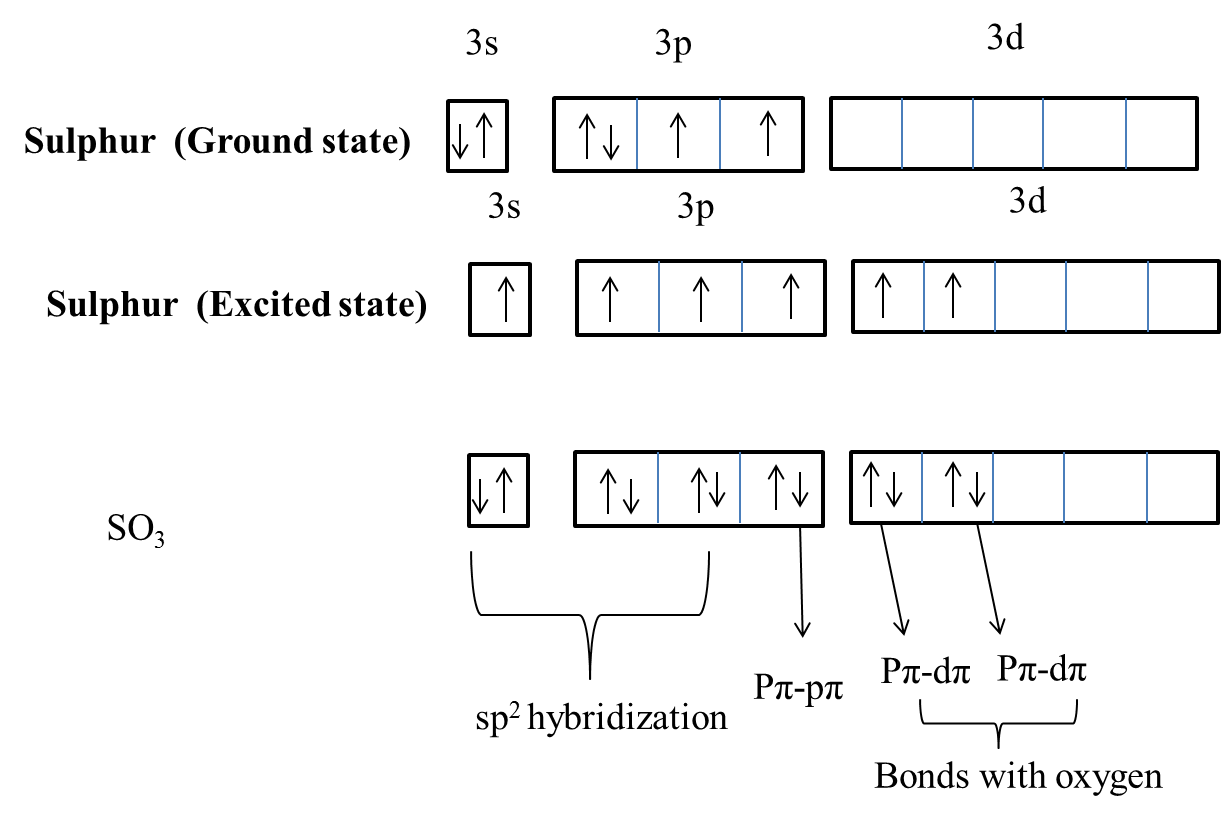
- We can see clearly in the above picture that there is one $p\pi -p\pi $ bond and there are two $p\pi -d\pi $ bonds in the structure of sulphur trioxide.
Therefore the number of $p\pi -d\pi $ bonds present in sulphur trioxide molecules are two.
Additional information:
- The structure of sulphur trioxide is as follows.
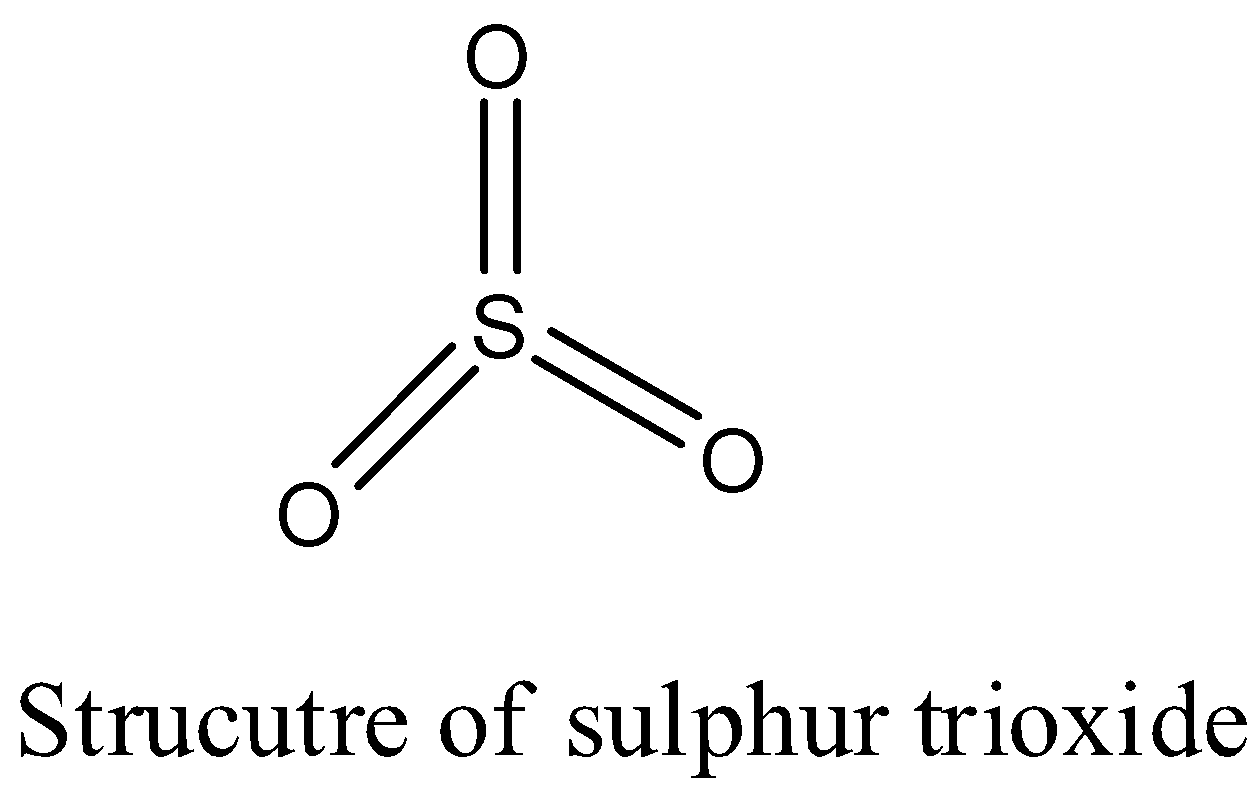
- In the above structure one oxygen atom is bonded with $p\pi -p\pi $ bond and the remaining two oxygen atoms bonded to sulphur with $p\pi -d\pi $ bonds.
- In the above structure there are three sigma bonds and three pi bonds.
- Sigma bonds are going to be formed by the overlapping of s and p orbitals and pi bonds are going to be formed by the overlapping of the p and d orbitals.
Note: The structure of the sulphur trioxide molecule is trigonal planar. In the sulphur trioxide molecule all the electrons are paired properly and no electrons are left with sulphur without forming bonds. We can see this in the above picture.
Complete step by step answer:
- The given molecule is sulphur trioxide ( $S{{O}_{3}}$ ).
- The ground state electronic configuration of the sulphur is as follows.
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{4}}\]
- The excited state electronic configuration of the sulphur is as follows.
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{1}}3{{p}^{3}}3{{d}^{2}}\]
- The electronic configuration of oxygen is as follows.
\[1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}\]
- The overlapping of orbitals between oxygen and sulphur atoms in sulphur trioxide is as follows.

- We can see clearly in the above picture that there is one $p\pi -p\pi $ bond and there are two $p\pi -d\pi $ bonds in the structure of sulphur trioxide.
Therefore the number of $p\pi -d\pi $ bonds present in sulphur trioxide molecules are two.
Additional information:
- The structure of sulphur trioxide is as follows.

- In the above structure one oxygen atom is bonded with $p\pi -p\pi $ bond and the remaining two oxygen atoms bonded to sulphur with $p\pi -d\pi $ bonds.
- In the above structure there are three sigma bonds and three pi bonds.
- Sigma bonds are going to be formed by the overlapping of s and p orbitals and pi bonds are going to be formed by the overlapping of the p and d orbitals.
Note: The structure of the sulphur trioxide molecule is trigonal planar. In the sulphur trioxide molecule all the electrons are paired properly and no electrons are left with sulphur without forming bonds. We can see this in the above picture.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




