
The oxidation numbers of the sulphur atoms in peroxymonosulfuric acid \[({H_2}S{O_5})\]and peroxodisulfuric acid\[({H_2}{S_2}{O_8})\] are respectively
A.\[ + 8\] and \[ + 7\]
B.\[ + 3\] and \[ + 3\]
C.\[ + 6\] and \[ + 6\]
D.\[ + 4\] and \[ + 6\]
Answer
575.4k+ views
Hint: We should be aware of the fact that the maximum oxidation state for sulphur in a compound is +6.
In general, we observe that oxo-acids of sulphur have \[ + 4\] and \[ + 6\] oxidation state. Since sulphur does not exhibit negative oxidation state in oxo acids, we can eliminate such options if mentioned in the question.
Formula used:
To solve such questions with higher accuracy, we should be well-versed with the structures of the compound to be dealt with.
Oxidation number is calculated on the basis of structure.
Complete step by step answer:
Before knowing the term oxidation number, we should be familiar with what oxidation and reduction means.
So, we will study it in the tabular form.
Reduction
Gain of hydrogen (electropositive element).
Loss of oxygen (electronegative element).
Gain of electrons.
Oxidation
Loss of hydrogen (electropositive element).
Gain of oxygen (electronegative element).
Loss of electrons.
An atom either gains or loses electrons in order to form a chemical bond with another atom.
This positive or negative value shows the effective charge of a particle (be it an atom or element) is called oxidation number.
Peroxomonosulphuric acid, commonly called Caro's acid, has the molecular formula\[{H_2}S{O_5}\]. It contains one peroxy linkage in which the oxidation state of oxygen is -1 instead of -2. The diagram is as shown below:
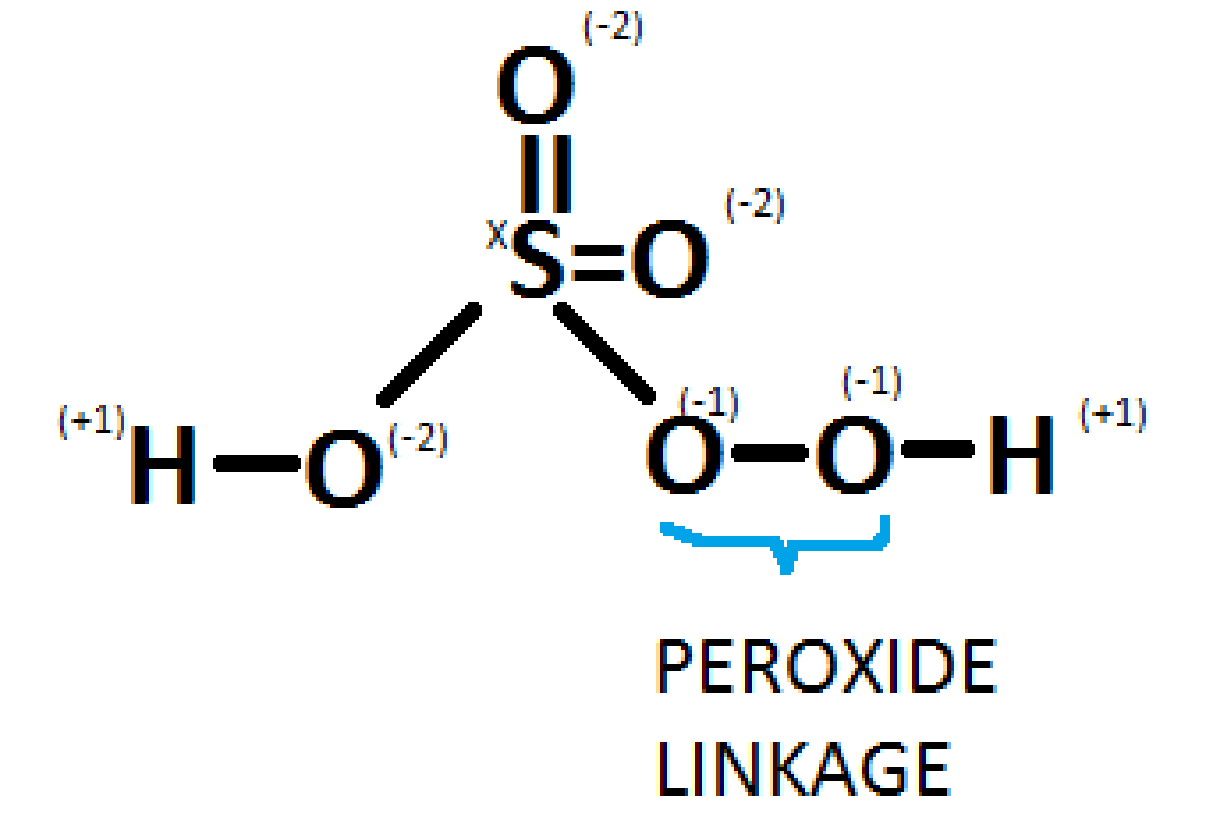
Solving the question for x,
We get
\[
x + 3( - 2) + 2( - 1) + 2(1) = 0 \\
\Rightarrow x + ( - 6) = 0 \\
\Rightarrow x = + 6. \\
\\
\]
Thus, the oxidation state for peroxymonosulfuric acid\[({H_2}S{O_5})\] is +6.
Now, we will look into a detailed discussion for peroxodisulfuric acid\[({H_2}{S_2}{O_8})\], also known as Marshall’s acid.
As we see a peroxy linkage in peroxymonosulfuric acid\[({H_2}S{O_5})\] , similarly when we draw the structure for\[{H_2}{S_2}{O_8}\], we get one peroxy linkage in which the oxidation state of oxygen is – 1 , rather than -2. Following is the structure for\[{H_2}{S_2}{O_8}\]
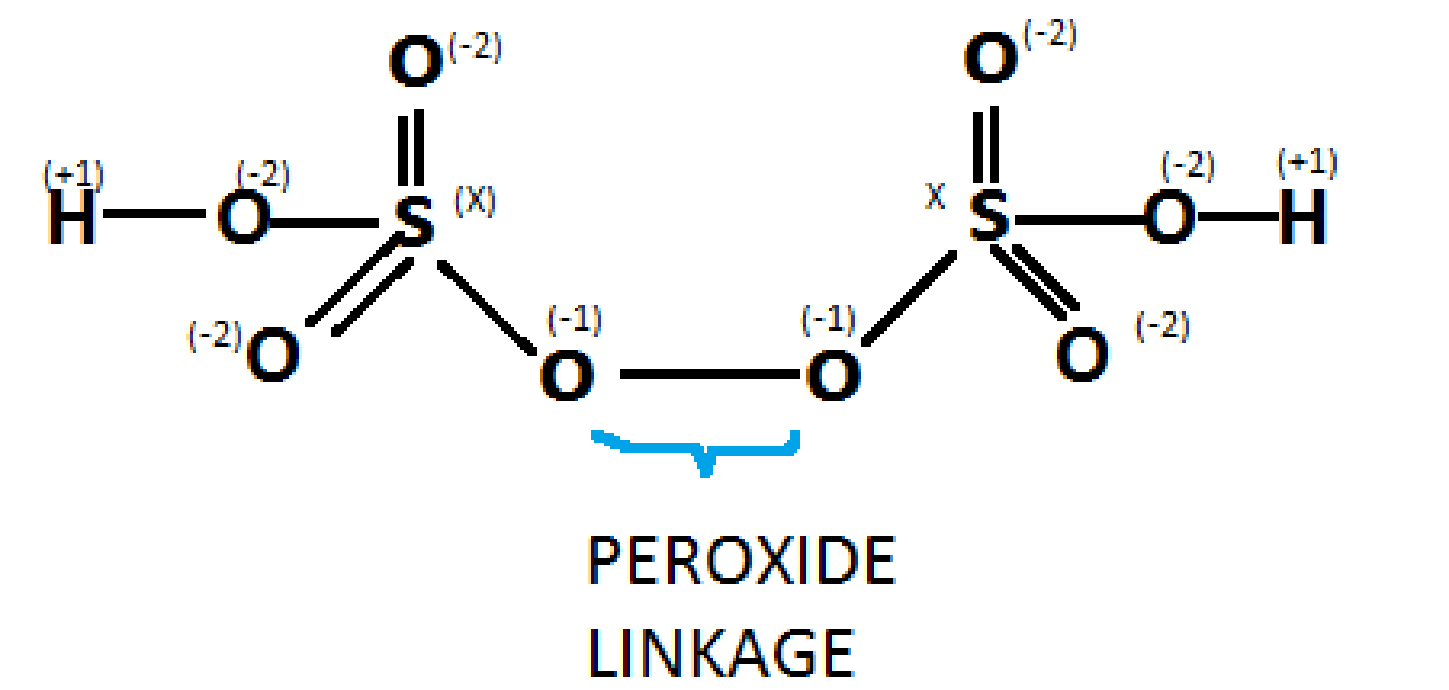
As we can see in the diagram,
We have to find a value of x in order to get the oxidation state of sulphur in \[{H_2}{S_2}{O_8}\]
Solving for x,
We deduce that:
\[
\;2x + 6( - 2) + 2( - 1) + 2(1) = 0 \\
\Rightarrow 2x + 2( - 6) = 0 \\
\Rightarrow x = + 6. \\
\\
\]
Therefore, C. option is the correct option for the given question.
Additional Information:
Oxo-acids of sulphur are \[s{p^3}\]hybridized.
\[{H_2}{S_2}{O_8}\] is a powerful oxidizing agent used in the industry.
\[{H_2}{S_{}}{O_5}\] is widely used in hydrometallurgy industries.
Note:
The oxidation number of a free element is zero.
An Interesting fact to note about the sulphur;
\[Cyclo - undeca sulphur monoxide,{S_{11}}O\], has the lowest oxidation number for sulphur.
In general, we observe that oxo-acids of sulphur have \[ + 4\] and \[ + 6\] oxidation state. Since sulphur does not exhibit negative oxidation state in oxo acids, we can eliminate such options if mentioned in the question.
Formula used:
To solve such questions with higher accuracy, we should be well-versed with the structures of the compound to be dealt with.
Oxidation number is calculated on the basis of structure.
Complete step by step answer:
Before knowing the term oxidation number, we should be familiar with what oxidation and reduction means.
So, we will study it in the tabular form.
Reduction
Gain of hydrogen (electropositive element).
Loss of oxygen (electronegative element).
Gain of electrons.
Oxidation
Loss of hydrogen (electropositive element).
Gain of oxygen (electronegative element).
Loss of electrons.
An atom either gains or loses electrons in order to form a chemical bond with another atom.
This positive or negative value shows the effective charge of a particle (be it an atom or element) is called oxidation number.
Peroxomonosulphuric acid, commonly called Caro's acid, has the molecular formula\[{H_2}S{O_5}\]. It contains one peroxy linkage in which the oxidation state of oxygen is -1 instead of -2. The diagram is as shown below:

Solving the question for x,
We get
\[
x + 3( - 2) + 2( - 1) + 2(1) = 0 \\
\Rightarrow x + ( - 6) = 0 \\
\Rightarrow x = + 6. \\
\\
\]
Thus, the oxidation state for peroxymonosulfuric acid\[({H_2}S{O_5})\] is +6.
Now, we will look into a detailed discussion for peroxodisulfuric acid\[({H_2}{S_2}{O_8})\], also known as Marshall’s acid.
As we see a peroxy linkage in peroxymonosulfuric acid\[({H_2}S{O_5})\] , similarly when we draw the structure for\[{H_2}{S_2}{O_8}\], we get one peroxy linkage in which the oxidation state of oxygen is – 1 , rather than -2. Following is the structure for\[{H_2}{S_2}{O_8}\]

As we can see in the diagram,
We have to find a value of x in order to get the oxidation state of sulphur in \[{H_2}{S_2}{O_8}\]
Solving for x,
We deduce that:
\[
\;2x + 6( - 2) + 2( - 1) + 2(1) = 0 \\
\Rightarrow 2x + 2( - 6) = 0 \\
\Rightarrow x = + 6. \\
\\
\]
Therefore, C. option is the correct option for the given question.
Additional Information:
Oxo-acids of sulphur are \[s{p^3}\]hybridized.
\[{H_2}{S_2}{O_8}\] is a powerful oxidizing agent used in the industry.
\[{H_2}{S_{}}{O_5}\] is widely used in hydrometallurgy industries.
Note:
The oxidation number of a free element is zero.
An Interesting fact to note about the sulphur;
\[Cyclo - undeca sulphur monoxide,{S_{11}}O\], has the lowest oxidation number for sulphur.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




