
The pH of a carbonated drink is ___________.
(A) less than 7
(B) more than 7
(C) equal to 7
(D) approximately $ 7.8 $
Answer
548.4k+ views
Hint: pH is a scale used to identify acidity or basicity of an aqueous solution. Acidic solutions have lower pH value and basic or alkaline solutions have a higher pH value.
Complete Step by step solution:
The pH can be less than zero for very strong acids and greater than fourteen for very strong bases. At $ {25^ \circ } $ C, solutions with pH less than 7 are acidic and solutions with greater than 7 pH are basic. More precisely pH is negative of base 10 logarithm of hydrogen ion that is $ {H^ + } $ concentration. The concept of pH was introduced by Danish chemist Soren Dedec in the year 1909.
In carbonated drinks, there is a presence of carbon dioxide and water. Carbon dioxide reacts with water as the following reaction,
$ C{O_2} + {H_2}O \to {H_2}C{O_3} $
where $ C{O_2} $ is carbon dioxide, $ {H_2}O $ is water and $ {H_2}C{O_3} $ is carbonic acid.
Carbonic acid formed here is an acid and it releases $ {H^ + } $ ion and forms bicarbonate ion.
$ {H_2}C{O_3} \to {H^ + } + HC{O_3}^ - $
As carbonic acid releases $ {H^ + } $ ion, pH of carbonic acid decreases. And it becomes less than 7 due to an increase in concentration of $ {H^ + } $ ion. The pH of carbonated drinks is around 3 to 4.
Note:
In ocean acidification, carbonic acid is an important component. Its structure is
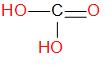
Carbonic acid plays an important role in the bicarbonate buffer system and it is used to maintain acid base homeostasis. Carbonic acid is also widely used in production of soft drinks and other bubbly beverages.
Complete Step by step solution:
The pH can be less than zero for very strong acids and greater than fourteen for very strong bases. At $ {25^ \circ } $ C, solutions with pH less than 7 are acidic and solutions with greater than 7 pH are basic. More precisely pH is negative of base 10 logarithm of hydrogen ion that is $ {H^ + } $ concentration. The concept of pH was introduced by Danish chemist Soren Dedec in the year 1909.
In carbonated drinks, there is a presence of carbon dioxide and water. Carbon dioxide reacts with water as the following reaction,
$ C{O_2} + {H_2}O \to {H_2}C{O_3} $
where $ C{O_2} $ is carbon dioxide, $ {H_2}O $ is water and $ {H_2}C{O_3} $ is carbonic acid.
Carbonic acid formed here is an acid and it releases $ {H^ + } $ ion and forms bicarbonate ion.
$ {H_2}C{O_3} \to {H^ + } + HC{O_3}^ - $
As carbonic acid releases $ {H^ + } $ ion, pH of carbonic acid decreases. And it becomes less than 7 due to an increase in concentration of $ {H^ + } $ ion. The pH of carbonated drinks is around 3 to 4.
Note:
In ocean acidification, carbonic acid is an important component. Its structure is

Carbonic acid plays an important role in the bicarbonate buffer system and it is used to maintain acid base homeostasis. Carbonic acid is also widely used in production of soft drinks and other bubbly beverages.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




