
The pressure (P) after mixing is
(A) 5 atm
(B) 2.5 atm
(C) 2 atm
(D) 4 atm

Answer
573.6k+ views
Hint: To calculate the total pressure of the gas, Dalton law of partial pressure is applied. The pressure in the third chamber is the total pressure which is the sum of both the pressure pressure exerted by two chambers
Complete step by step answer:
Given,
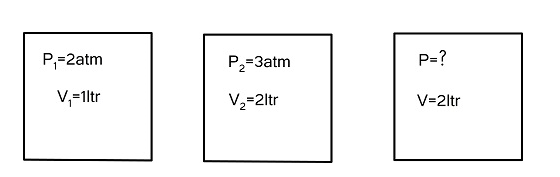
${P_1}$ is 2atm
${P_2}$ is 3atm
${V_1}$ is 1 ltr
${V_2}$ is 2 ltr
${V_{Total}}$ is 2 ltr
The Dalton law of partial pressure states that the total pressure exerted by the gaseous mixture is equal to the sum total of the individual partial pressure exerted by the gases molecule.
Example: There are two gases molecule (gas A and gas B) present having individual value of partial pressure. The total pressure exerted by the mixture of gas A and gas B is equal to the sum of the individual partial pressure exerted by both gas A and B.
The pressure exerted by the individual gas molecule present in a mixture of gases is known as partial pressure.
Dalton's law formula is given as shown below.
${P_{total}} = {P_1} + {P_2} + {P_3}$ ${P_n}$
The formula to calculate the total partial pressure is shown below.
${P_{total}} = {P_1} + {P_2}$
${P_{total}} = 2 + 3$
$\Rightarrow {P_{total}} = 5$
Thus, the total pressure after mixing of two gases present in the chamber is 5 atm.
So, the correct answer is Option A.
Note: Dalton’s law of partial pressure helps in determining the pressure of dry gas only. Unless the individual gases present do not react chemically with each other they do not affect the pressure.
Complete step by step answer:
Given,
${P_1}$ is 2atm
${P_2}$ is 3atm
${V_1}$ is 1 ltr
${V_2}$ is 2 ltr
${V_{Total}}$ is 2 ltr
The Dalton law of partial pressure states that the total pressure exerted by the gaseous mixture is equal to the sum total of the individual partial pressure exerted by the gases molecule.
Example: There are two gases molecule (gas A and gas B) present having individual value of partial pressure. The total pressure exerted by the mixture of gas A and gas B is equal to the sum of the individual partial pressure exerted by both gas A and B.
The pressure exerted by the individual gas molecule present in a mixture of gases is known as partial pressure.
Dalton's law formula is given as shown below.
${P_{total}} = {P_1} + {P_2} + {P_3}$ ${P_n}$
The formula to calculate the total partial pressure is shown below.
${P_{total}} = {P_1} + {P_2}$
${P_{total}} = 2 + 3$
$\Rightarrow {P_{total}} = 5$
Thus, the total pressure after mixing of two gases present in the chamber is 5 atm.
So, the correct answer is Option A.
Note: Dalton’s law of partial pressure helps in determining the pressure of dry gas only. Unless the individual gases present do not react chemically with each other they do not affect the pressure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




