
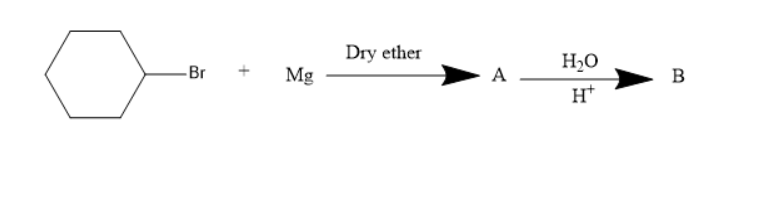
The product $B$ in the following chemical reaction is:




Answer
501.6k+ views
Hint: Cycloalkanes are compounds containing carbon and hydrogen atoms only arranged in the cyclic or ring structure. Cyclo alkyl halides contain a halogen atom in addition to the cyclic hydrocarbon, which upon treatment with magnesium forms a Grignard reagent. Grignard reagent on hydrolysis forms alkanes.
Complete answer:
Alkanes are the saturated hydrocarbons consisting of only carbon and hydrogen atoms. These compounds contain only single bonds. The alkanes that exist in the cyclic or ring structure can be called cyclo alkanes.
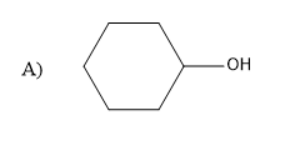
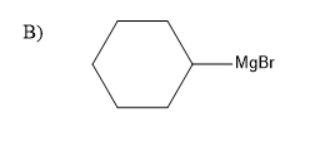
The presence of a halogen atom on cyclo alkanes can be called cyclo alkyl halides. Given compound is a cyclo hexyl bromide. Upon treatment of the magnesium with cyclohexyl Bromide it gives a Grignard reagent. Grignard reagent is an organometallic compound, it contains an alkyl part, halogen and metal.
Thus, the Grignard reagent is formed by treating magnesium with cyclohexyl bromide. The Grignard reagent on hydrolysis forms an alkane and a compound $ MgBrOH $ .
The chemical reaction for the given reaction will be as follows:
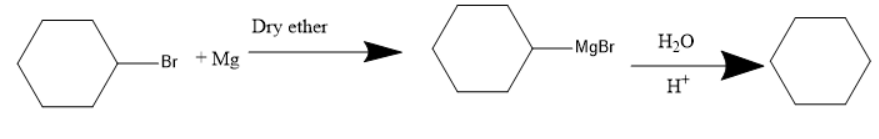
The chemical reaction involved in the given question will form the above products.
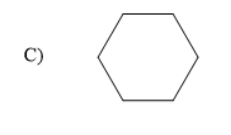
Therefore, the product A is a Grignard reagent and the compound B is cyclohexane. So, option (C) is correct.
Note:
Cyclohexane is a cycloalkane with the molecular formula of $ {C_6}{H_{12}} $ , the general molecular formula of cyclo alkanes is $ {C_n}{H_{2n}} $ , where n is the number of carbon atoms. Cyclo alkanes are the functional isomers of alkenes. The Grignard reagent upon hydrolysis forms alkanes as the alkyl part in the Grignard reagent has a negative charge.
Complete answer:
Alkanes are the saturated hydrocarbons consisting of only carbon and hydrogen atoms. These compounds contain only single bonds. The alkanes that exist in the cyclic or ring structure can be called cyclo alkanes.
The presence of a halogen atom on cyclo alkanes can be called cyclo alkyl halides. Given compound is a cyclo hexyl bromide. Upon treatment of the magnesium with cyclohexyl Bromide it gives a Grignard reagent. Grignard reagent is an organometallic compound, it contains an alkyl part, halogen and metal.
Thus, the Grignard reagent is formed by treating magnesium with cyclohexyl bromide. The Grignard reagent on hydrolysis forms an alkane and a compound $ MgBrOH $ .
The chemical reaction for the given reaction will be as follows:

The chemical reaction involved in the given question will form the above products.
Therefore, the product A is a Grignard reagent and the compound B is cyclohexane. So, option (C) is correct.
Note:
Cyclohexane is a cycloalkane with the molecular formula of $ {C_6}{H_{12}} $ , the general molecular formula of cyclo alkanes is $ {C_n}{H_{2n}} $ , where n is the number of carbon atoms. Cyclo alkanes are the functional isomers of alkenes. The Grignard reagent upon hydrolysis forms alkanes as the alkyl part in the Grignard reagent has a negative charge.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




