
The product of the reaction of ethyl orthoformate, \[HC{(OEt)_3}\] with one equivalent of $C{H_3}MgBr$ followed by the hydrolysis would be:
A.$C{H_3}CH{(OEt)_2}$
B.$C{H_3}CHO$
C.$C{H_3}C{(OEt)_3}$
D.No reaction takes place
Answer
548.1k+ views
Hint: Three ethoxy groups are not stable on single carbon so before reaction with any other reactant ethyl orthoformate will lose one of its ethoxy group and will undergo reaction with the given organometallic reagent.
Complete step by step answer:
We are given ethyl orthoformate which have three ethoxy groups on a single atom which is highly unstable, so before reacting further with anything ethyl orthoformate will remove one of its ethoxy group as shown below in the reaction. In the reaction there is a formation of ethoxy anion which abstract the ethyl group present on the doubly bonded oxygen and to neutralize the system and ethyl ether is formed as a by-product.
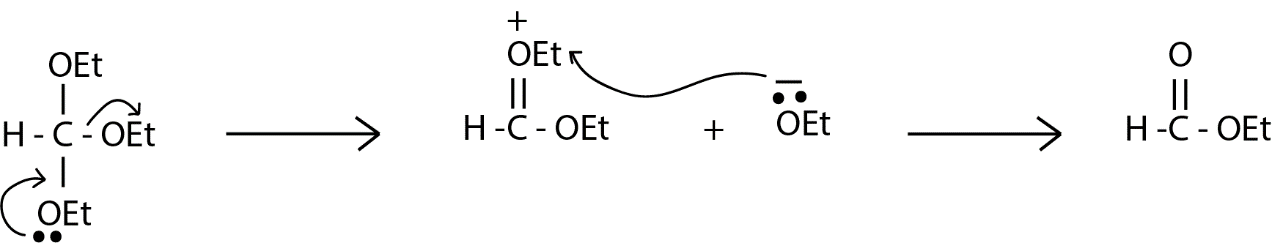
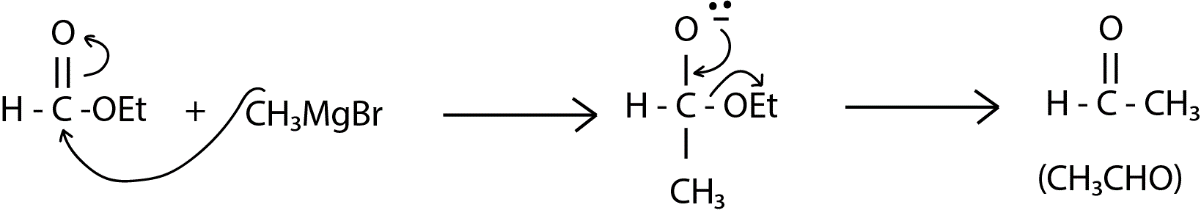
Now, our next step is the reaction with the given organometallic compound \[C{H_3}MgBr\] in which the magnesium carries a partial positive charge and the methyl group has a negative charge which can attack on the carbonyl carbon. Carbonyl carbon is an electrophilic center because of the oxygen and the double bond, and $ - MgBr$part will go and attach to the oxygen atom. After this is formed as shown below in reaction hydrolysis happens in which the metal is removed and the system or the oxygen will now have a proton on it. This might be considered as the final product but it is not because in the system we have a ethoxy group which is a good leaving group and the oxygen in $ - {O^ - }$ can give its electron and can form oxygen carbon double bond and finally we have a formation of acetaldehyde or ethanal as given below:
Note:
In this reaction two things should be taken care of first is that one single carbon cannot have three ethoxy groups and second that if oxygen can give lone pair and there is leaving group then the carbon will prefer to remove the leaving group and form a double bond with oxygen.
Complete step by step answer:
We are given ethyl orthoformate which have three ethoxy groups on a single atom which is highly unstable, so before reacting further with anything ethyl orthoformate will remove one of its ethoxy group as shown below in the reaction. In the reaction there is a formation of ethoxy anion which abstract the ethyl group present on the doubly bonded oxygen and to neutralize the system and ethyl ether is formed as a by-product.
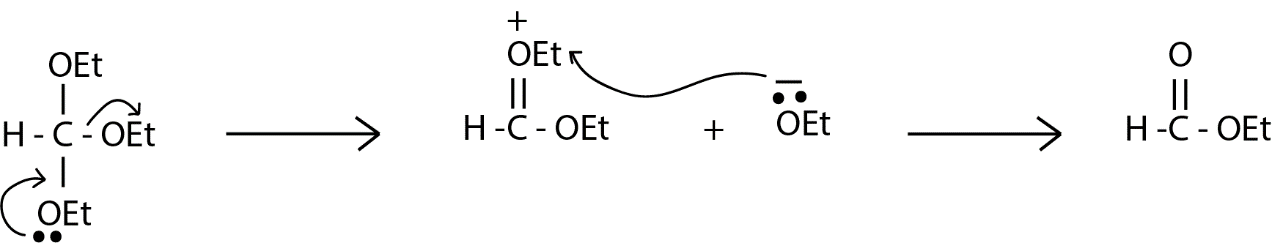
Now, our next step is the reaction with the given organometallic compound \[C{H_3}MgBr\] in which the magnesium carries a partial positive charge and the methyl group has a negative charge which can attack on the carbonyl carbon. Carbonyl carbon is an electrophilic center because of the oxygen and the double bond, and $ - MgBr$part will go and attach to the oxygen atom. After this is formed as shown below in reaction hydrolysis happens in which the metal is removed and the system or the oxygen will now have a proton on it. This might be considered as the final product but it is not because in the system we have a ethoxy group which is a good leaving group and the oxygen in $ - {O^ - }$ can give its electron and can form oxygen carbon double bond and finally we have a formation of acetaldehyde or ethanal as given below:

Note:
In this reaction two things should be taken care of first is that one single carbon cannot have three ethoxy groups and second that if oxygen can give lone pair and there is leaving group then the carbon will prefer to remove the leaving group and form a double bond with oxygen.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




