
The products of (A) on ozonolysis followed by reduction with $LAH$ are:
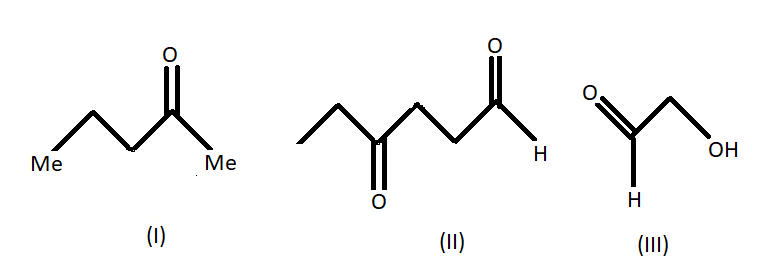
A.
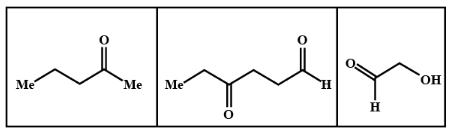
B.
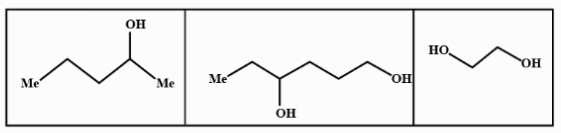
C.
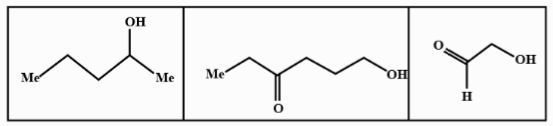
D.





Answer
558.9k+ views
Hint: We have studied Ozonolysis or ozonolysis- reduction can be defined as the treatment of any compound with ozone followed by a suitable reducing agent so that complex double bond containing compounds can be broken down into more easy products.
Complete step by step answer:
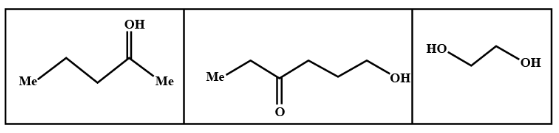
We know that LAH or lithium aluminium hydride is one of the strongest reducing agents. It reduces any $C = O$ containing functional group to an alcohol . In the given compound (I) , (II) and (III) after reduction they will reduce the carboxylic group to alcohol. Here given compounds contain aldehyde and ketone groups which will be reduced into alcohol on treatment with lithium aluminium hydride.
Oxidative cleavage of carbon -carbon double bond with ozone as an oxidative agent is called ozonolysis. This reaction used to perform common organic solvents such as methanol, acetone or dichloromethane at a specific temperature. Ozonolysis takes place through ozonide intermediate followed by work up results in the formation of respective products. Strong reducing work up such as lithium aluminium hydride produces alcohol. The products of (A) on ozonolysis followed by reduction with $LAH$ are given below:
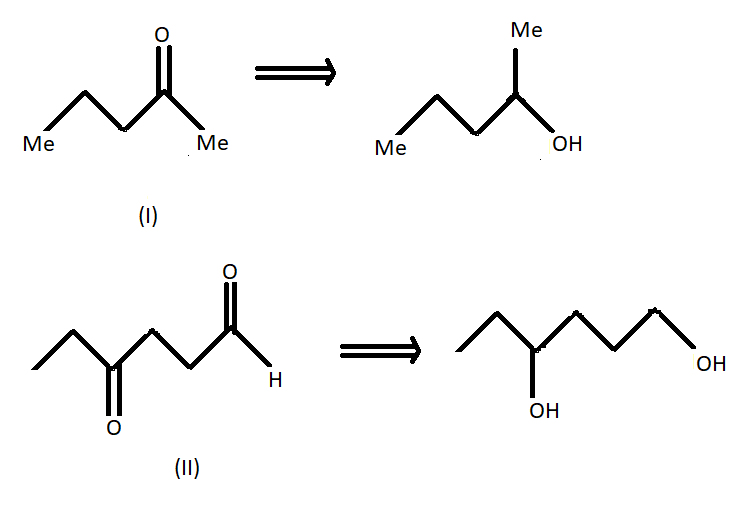
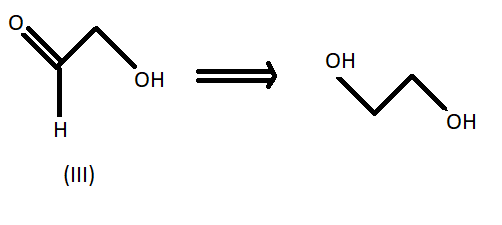
Hence option B shows all the above products so it is the correct answer to this problem so the product (A) on ozonolysis followed by reduction with LAH are represented by the compounds given in the option (B).
Therefore, the correct answer is “Option (B)”.
Note: We have approached this reaction by reducing $C = O$ containing function group to an alcohol with lithium aluminium hydride. So we have to check in the given option that the $C = O$ containing functional group should be changed in the alcoholic group.
Complete step by step answer:
We know that LAH or lithium aluminium hydride is one of the strongest reducing agents. It reduces any $C = O$ containing functional group to an alcohol . In the given compound (I) , (II) and (III) after reduction they will reduce the carboxylic group to alcohol. Here given compounds contain aldehyde and ketone groups which will be reduced into alcohol on treatment with lithium aluminium hydride.
Oxidative cleavage of carbon -carbon double bond with ozone as an oxidative agent is called ozonolysis. This reaction used to perform common organic solvents such as methanol, acetone or dichloromethane at a specific temperature. Ozonolysis takes place through ozonide intermediate followed by work up results in the formation of respective products. Strong reducing work up such as lithium aluminium hydride produces alcohol. The products of (A) on ozonolysis followed by reduction with $LAH$ are given below:


Hence option B shows all the above products so it is the correct answer to this problem so the product (A) on ozonolysis followed by reduction with LAH are represented by the compounds given in the option (B).
Therefore, the correct answer is “Option (B)”.
Note: We have approached this reaction by reducing $C = O$ containing function group to an alcohol with lithium aluminium hydride. So we have to check in the given option that the $C = O$ containing functional group should be changed in the alcoholic group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




