
The reaction of Benzenediazonium chloride with aniline yields yellow dye. The name of the yellow dye is:
A. p-hydroxyazobenzene
B. p- aminoazebenzene
C. p-nitroazobenzene
D. o-nitroazobenzene
Answer
546.3k+ views
Hint: Coupling reaction between benzene diazonium chlorides with aniline in acidic medium takes place to give yellow colour. In this reaction the nitrogen ${N_2}$ in the diazonium ion is lost and forms the bridge between two benzene rings forming an electrophilic substitution reaction.
Complete step-by-step answer:A Coupling reaction is an organic reaction between a diazonium compound and another aromatic compound. When liquid phenylamine (aniline) is added to the cold solution of benzene diazonium chloride (colourless) reaction mixture is shaken vigorously and a yellow colour solvent is produced.
The coupling occurs between the terminal diazonium nitrogen of the benzene diazonium ion and the para-position of aniline. Diazotization takes place by replacement of hydrogen in aniline and the chlorine on benzene diazonium chloride and forms a complex of azo-derivative.
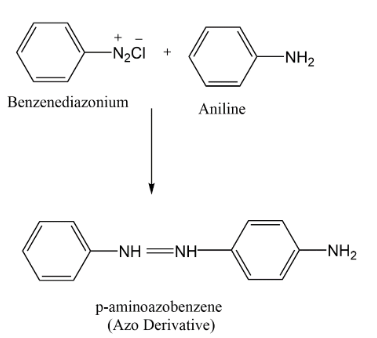
These Azo products have conjugate systems joining both the aromatic compounds with $(N = N)$ . Azo compounds contain a highly delocalised system of electrons which takes in both benzene rings and the two nitrogen atoms bridging the rings. These Azo derived compounds are mostly coloured and are known as Azo-dyes.
Hence the correct answer is option (B) P- Aminoazobenzene.
Note: The coupling reaction between benzene diazonium chlorides with aniline is a coupling reaction in which retention of diazo groups remains whereas conjugation of aromatic rings takes place to form azo derivative compounds which are coloured in nature.
During the process some wavelengths are absorbed by the delocalised electrons. The group of electrons which get absorbed are called Chromophore. The colour which we see is developed in the reaction is due to the non-absorbed wavelength of the electrons.
Complete step-by-step answer:A Coupling reaction is an organic reaction between a diazonium compound and another aromatic compound. When liquid phenylamine (aniline) is added to the cold solution of benzene diazonium chloride (colourless) reaction mixture is shaken vigorously and a yellow colour solvent is produced.
The coupling occurs between the terminal diazonium nitrogen of the benzene diazonium ion and the para-position of aniline. Diazotization takes place by replacement of hydrogen in aniline and the chlorine on benzene diazonium chloride and forms a complex of azo-derivative.

These Azo products have conjugate systems joining both the aromatic compounds with $(N = N)$ . Azo compounds contain a highly delocalised system of electrons which takes in both benzene rings and the two nitrogen atoms bridging the rings. These Azo derived compounds are mostly coloured and are known as Azo-dyes.
Hence the correct answer is option (B) P- Aminoazobenzene.
Note: The coupling reaction between benzene diazonium chlorides with aniline is a coupling reaction in which retention of diazo groups remains whereas conjugation of aromatic rings takes place to form azo derivative compounds which are coloured in nature.
During the process some wavelengths are absorbed by the delocalised electrons. The group of electrons which get absorbed are called Chromophore. The colour which we see is developed in the reaction is due to the non-absorbed wavelength of the electrons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




