
The reaction of methyl magnesium iodide with acetone followed by hydrolysis, gives secondary alcohol.
a) True
b) False
Answer
546.6k+ views
Hint : In the metal – carbon bond the electronegativity of the alkyl group is maximum. Thus the alkyl group acts as a nucleophile and attacks the most electrophilic centre which in this case is a carbonyl group in acetone.
Complete Step By Step Answer:
Grignard reagents are the organometallic compound where the metal carbon bond is present. This method is the most convenient for the preparation of alcohols using the aldehydes and ketones.
In this reagent i.e. Grignard reagent (organomagnesium halide), the alkyl group acts as a nucleophile. As we know the carbonyl carbon is electron deficient due to the presence of electronegative atom oxygen, and acts as a good electrophilic centre.
When the organomagnesium halide attacks the electrophilic centre followed by hydrolysis, an additional product is obtained which results in the formation of alcohol.
Now there are some differences when we use Grignard reagent with aldehydes and with ketones. By selecting a proper Grignard reagent we can easily form primary, secondary and tertiary alcohols in reaction with aldehydes and ketones.
When formaldehyde reacts with methyl magnesium iodide, followed by hydrolysis, the formation of primary alcohol takes place.
When aldehydes other than formaldehyde reacts with methyl magnesium iodide, followed by hydrolysis, the formation of secondary alcohol takes place.
When methyl magnesium iodide reacts with acetone (ketone) followed by hydrolysis formation of tertiary alcohols takes place. And not the secondary alcohols. For e.g. in the reaction of methyl magnesium iodide with acetone which is a ketone and further followed by hydrolysis results in the formation of $ 2 - Methylpropan - 2 - ol $ that is a tertiary alcohol.
The mechanism can be drawn as follows-
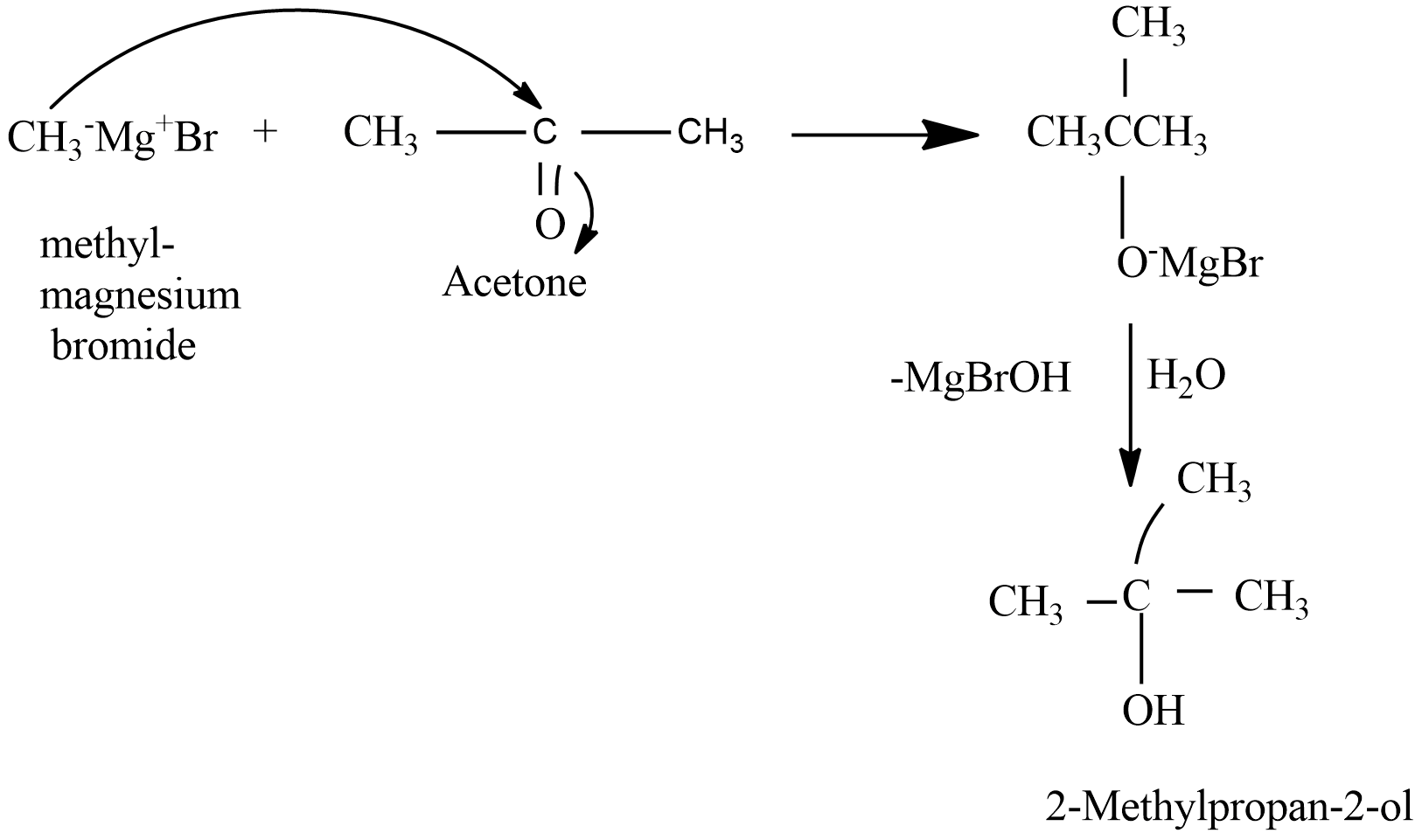
Hence the given statement is (b) False.
Note :
Alcohols can also be formed when the Grignard reagent reacts with epoxides. In this process ring opening of epoxide takes place followed by hydrolysis. Then the product can be obtained as alcohol. By this method with substituted epoxides we can form all primary, secondary and tertiary alcohols.
Complete Step By Step Answer:
Grignard reagents are the organometallic compound where the metal carbon bond is present. This method is the most convenient for the preparation of alcohols using the aldehydes and ketones.
In this reagent i.e. Grignard reagent (organomagnesium halide), the alkyl group acts as a nucleophile. As we know the carbonyl carbon is electron deficient due to the presence of electronegative atom oxygen, and acts as a good electrophilic centre.
When the organomagnesium halide attacks the electrophilic centre followed by hydrolysis, an additional product is obtained which results in the formation of alcohol.
Now there are some differences when we use Grignard reagent with aldehydes and with ketones. By selecting a proper Grignard reagent we can easily form primary, secondary and tertiary alcohols in reaction with aldehydes and ketones.
When formaldehyde reacts with methyl magnesium iodide, followed by hydrolysis, the formation of primary alcohol takes place.
When aldehydes other than formaldehyde reacts with methyl magnesium iodide, followed by hydrolysis, the formation of secondary alcohol takes place.
When methyl magnesium iodide reacts with acetone (ketone) followed by hydrolysis formation of tertiary alcohols takes place. And not the secondary alcohols. For e.g. in the reaction of methyl magnesium iodide with acetone which is a ketone and further followed by hydrolysis results in the formation of $ 2 - Methylpropan - 2 - ol $ that is a tertiary alcohol.
The mechanism can be drawn as follows-

Hence the given statement is (b) False.
Note :
Alcohols can also be formed when the Grignard reagent reacts with epoxides. In this process ring opening of epoxide takes place followed by hydrolysis. Then the product can be obtained as alcohol. By this method with substituted epoxides we can form all primary, secondary and tertiary alcohols.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




