
The shape of $I{F_4^-}$ will be:
A) Square planar
B) Tetrahedral
C) Pentagonal bipyramidal
D) Distorted tetrahedral
Answer
580.8k+ views
Hint: The compound given to us in the question is an interhalogen compound, iodine tetrafluoride. It exists in the form $IF_4^ - $. Let us now understand the shape of the polyhalide under VSEPR theory or valence shell electron pair repulsion theory.
Complete answer:
Before getting further into the shape of iodine tetrafluoride let us briefly understand VSEPR theory, it states that shape of molecule depends upon the number of valence shell electron pairs (bonded or non bonded ) around the central atom. These electrons tend to minimize repulsion and maximize distance between them. It is quite accurate to predict the geometry of p-block elements.
Hence to predict the shape of the molecule we have to predict the hybridization, then the shape of the molecule depends on the number of lone pairs and shared pairs of electrons. If only a shared pair of electrons is present then the shape is regular geometry. If an unshared pair of electrons are present then the shape of the molecule is distorted geometry.
We can calculate hybridization using the formula.
To calculate hybridization:
H = $\dfrac{1}{2}$ X (Number of Electrons in the valence orbital of the central atom) + No. of atoms attached to it + (Number of negative charge ). Now let us put the values. When we know that iodine has seven electrons in its valence orbital and four fluorine atoms are attached to it and as explained in the hint the charge on iodine tetrafluoride is -1. Therefore we get,
$H = \dfrac{1}{2} \times (7 + 4 + 1) = 6$
So the number of bonding electron pairs are 4 and the number of lone pairs of electrons are 2.
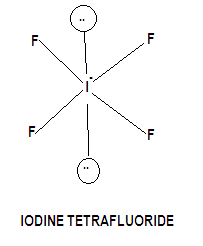
Structure of iodine tetrafluoride gets formed by three covalent bonds and one coordinate bond. So Hybridization = \[s{p^3}{d^2}\], the shape of the molecule is octahedral geometry, but the geometry of the atom takes a square planar shape is due to iodine carries two lone pairs of electrons, one above the plane and one below the plane hence the shape of the molecule is square planar.
Hence we can conclude from the above arguments that the correct option is A.
Note: Mixing of atomic orbital of the same atom to form a new orbital of the same shape, size, number, energy orbital is known as hybridization. In \[s{p^3}{d^2}\] hybridization mixing of one S orbital, two P orbital and two d orbitals to form 6 hybrid orbitals.
Complete answer:
Before getting further into the shape of iodine tetrafluoride let us briefly understand VSEPR theory, it states that shape of molecule depends upon the number of valence shell electron pairs (bonded or non bonded ) around the central atom. These electrons tend to minimize repulsion and maximize distance between them. It is quite accurate to predict the geometry of p-block elements.
Hence to predict the shape of the molecule we have to predict the hybridization, then the shape of the molecule depends on the number of lone pairs and shared pairs of electrons. If only a shared pair of electrons is present then the shape is regular geometry. If an unshared pair of electrons are present then the shape of the molecule is distorted geometry.

We can calculate hybridization using the formula.
To calculate hybridization:
H = $\dfrac{1}{2}$ X (Number of Electrons in the valence orbital of the central atom) + No. of atoms attached to it + (Number of negative charge ). Now let us put the values. When we know that iodine has seven electrons in its valence orbital and four fluorine atoms are attached to it and as explained in the hint the charge on iodine tetrafluoride is -1. Therefore we get,
$H = \dfrac{1}{2} \times (7 + 4 + 1) = 6$
So the number of bonding electron pairs are 4 and the number of lone pairs of electrons are 2.
Structure of iodine tetrafluoride gets formed by three covalent bonds and one coordinate bond. So Hybridization = \[s{p^3}{d^2}\], the shape of the molecule is octahedral geometry, but the geometry of the atom takes a square planar shape is due to iodine carries two lone pairs of electrons, one above the plane and one below the plane hence the shape of the molecule is square planar.
Hence we can conclude from the above arguments that the correct option is A.
Note: Mixing of atomic orbital of the same atom to form a new orbital of the same shape, size, number, energy orbital is known as hybridization. In \[s{p^3}{d^2}\] hybridization mixing of one S orbital, two P orbital and two d orbitals to form 6 hybrid orbitals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




