
The ‘spin only’ magnetic moment of $N{i^{2 + }}$ in aqueous solution would be:
A. $\sqrt 6 BM$
B. $\sqrt {15} BM$
C. $\sqrt 2 BM$
D. $\sqrt 8 BM$
Answer
584.7k+ views
Hint: The net magnetic moment of an atom/ion is the vector sum of its orbital and spin magnetic moments. We can easily calculate the spin only magnetic moment using the formula ${u_{spin}} = \sqrt {n(n + 2)} $ where n is the number of unpaired electrons present in an atom or ion. Bohr magneton or BM is the unit of magnetic moment.
Complete step by step answer:
$Ni$ is present in $N{i^{2 + }}$ oxidation state as ${\left[ {Ni{{({H_2}O)}_6}} \right]^{2 + }}$
First of all, we know that Ni is a d-block element and ${H_2}O$ being an electron rich species (due to presence of two lone pairs on oxygen atoms) can easily form a coordinate compound.
We look, into the electronic configuration of $N{i^{2 + }}$ ion ${\left[ {Ni{{({H_2}O)}_6}} \right]^{2 + }}$
$Ni = {16^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^8}4{s^0}$
$N{i^{2 + }} = 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^8}4{s^0}$
Thus, according to valence and theory (VB+) water being a ligand form of a high spin complex with $N{i^{2 + }}$ and this can be as show below
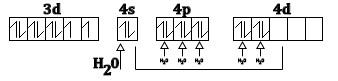
The electrons are filled according to Hund’s rule. Thus, the spin only magnetic moment can be easily calculated using the data given above. As we have seen there are two unpaired electrons in the complex compound so
${u_{spin}} = \sqrt {n(n + 2)} \,BM$
$ = \,\sqrt {2(2 + 2)} \,BM$
\[ = \,\sqrt 8 \,BM\]
Where, BM= Bohr magneton
Hence, option (d) is the correct option.
Note:
Student can also use an alternative method to calculate the spin only magnetic moment of the atom or ion by using a formula
${u_{spin}} = \,\sqrt {4s(s + 1)} \,BM$
Where, s= angular spin moment quantum number.
For $N{i^{2 + }}$ with two unpaired electrons
$s = \dfrac{1}{2} + \dfrac{1}{2} = 1$
Therefore, ${u_{spin}} = \sqrt {4 \times 1(1 + 1)} \,BM$
$ \Rightarrow \,\sqrt 8 \,BM$ which is the required solution.
Complete step by step answer:
$Ni$ is present in $N{i^{2 + }}$ oxidation state as ${\left[ {Ni{{({H_2}O)}_6}} \right]^{2 + }}$
First of all, we know that Ni is a d-block element and ${H_2}O$ being an electron rich species (due to presence of two lone pairs on oxygen atoms) can easily form a coordinate compound.
We look, into the electronic configuration of $N{i^{2 + }}$ ion ${\left[ {Ni{{({H_2}O)}_6}} \right]^{2 + }}$
$Ni = {16^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^8}4{s^0}$
$N{i^{2 + }} = 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}3{d^8}4{s^0}$
Thus, according to valence and theory (VB+) water being a ligand form of a high spin complex with $N{i^{2 + }}$ and this can be as show below

The electrons are filled according to Hund’s rule. Thus, the spin only magnetic moment can be easily calculated using the data given above. As we have seen there are two unpaired electrons in the complex compound so
${u_{spin}} = \sqrt {n(n + 2)} \,BM$
$ = \,\sqrt {2(2 + 2)} \,BM$
\[ = \,\sqrt 8 \,BM\]
Where, BM= Bohr magneton
Hence, option (d) is the correct option.
Note:
Student can also use an alternative method to calculate the spin only magnetic moment of the atom or ion by using a formula
${u_{spin}} = \,\sqrt {4s(s + 1)} \,BM$
Where, s= angular spin moment quantum number.
For $N{i^{2 + }}$ with two unpaired electrons
$s = \dfrac{1}{2} + \dfrac{1}{2} = 1$
Therefore, ${u_{spin}} = \sqrt {4 \times 1(1 + 1)} \,BM$
$ \Rightarrow \,\sqrt 8 \,BM$ which is the required solution.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




