
The structure of isoprene is:
a.) \[C{{H}_{3}}-CH=C=C{{H}_{2}}\]
b.) \[C{{H}_{2}}=C(C{{H}_{3}})-CH=C{{H}_{2}}\]
c.) \[CH\equiv C-C(C{{H}_{3}})=C{{H}_{2}}\]
d.) \[C{{H}_{2}}=C(C{{H}_{3}})-C{{H}_{2}}-CH=C{{H}_{2}}\]
Answer
595.5k+ views
Hint: "Iso" is a prefix, used when all carbons except one form a continuous chain. This one carbon is part of an isopropyl group at the second carbon of the chain. “Iso” sometimes also indicates that the molecule is a constitutional isomer of another molecule with a common name.
Complete step by step solution:
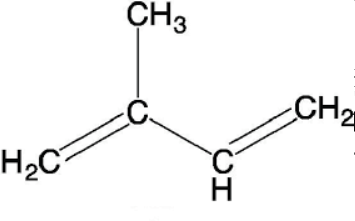
Isoprene is an unsaturated penta-hydrocarbon. IUPAC name of isoprene is 2-methyl-1,3-butadiene. It is a colorless, volatile liquid hydrocarbon having petroleum-like odor. Its chemical formula is \[{{C}_{5}}{{H}_{8}}\], structure is given as \[C{{H}_{2}}=C(C{{H}_{3}})-CH=C{{H}_{2}}\].
Additional Information:
Isoprene reacts very rapidly with hydroxyl radicals, reducing the oxidizing capacity in the atmosphere. This leads to an extended lifetime of greenhouse gases, methane, thus increasing global warming.
Nitric oxide if present in high concentrations, reacts with isoprene and produces nitrogen dioxide. And the formed nitrogen dioxide via photolysis, increases the amount of ozone, which is a greenhouse gas.
Oxidation of isoprene in the atmosphere forms secondary organic aerosols that cause scattering of the sunlight.
Isoprene is an important commodity chemical, currently produced from petroleum, with the potential to serve as an aviation fuel and a feedstock for numerous products, including its polymer, rubber. It is also used to give flavors and fragrances of essential oils and other substances derived from plants.
So, the correct answer is (b).
Note: The precise reactions with Nitric oxide and its effects depend on environmental conditions, such as light intensity and nitrogen oxide concentrations.
Complete step by step solution:
Isoprene is an unsaturated penta-hydrocarbon. IUPAC name of isoprene is 2-methyl-1,3-butadiene. It is a colorless, volatile liquid hydrocarbon having petroleum-like odor. Its chemical formula is \[{{C}_{5}}{{H}_{8}}\], structure is given as \[C{{H}_{2}}=C(C{{H}_{3}})-CH=C{{H}_{2}}\].

Additional Information:
Isoprene reacts very rapidly with hydroxyl radicals, reducing the oxidizing capacity in the atmosphere. This leads to an extended lifetime of greenhouse gases, methane, thus increasing global warming.
Nitric oxide if present in high concentrations, reacts with isoprene and produces nitrogen dioxide. And the formed nitrogen dioxide via photolysis, increases the amount of ozone, which is a greenhouse gas.
Oxidation of isoprene in the atmosphere forms secondary organic aerosols that cause scattering of the sunlight.
Isoprene is an important commodity chemical, currently produced from petroleum, with the potential to serve as an aviation fuel and a feedstock for numerous products, including its polymer, rubber. It is also used to give flavors and fragrances of essential oils and other substances derived from plants.
So, the correct answer is (b).
Note: The precise reactions with Nitric oxide and its effects depend on environmental conditions, such as light intensity and nitrogen oxide concentrations.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




