
The structure of Q will be
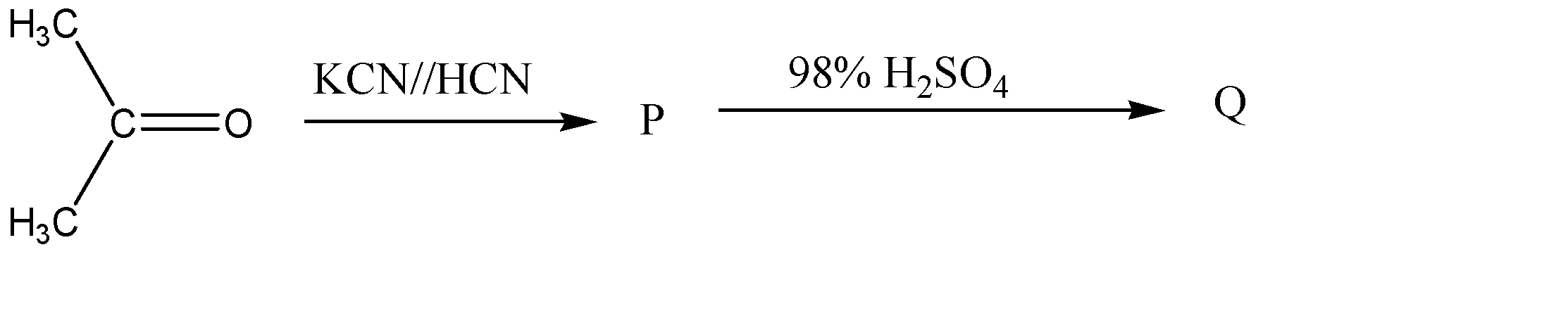
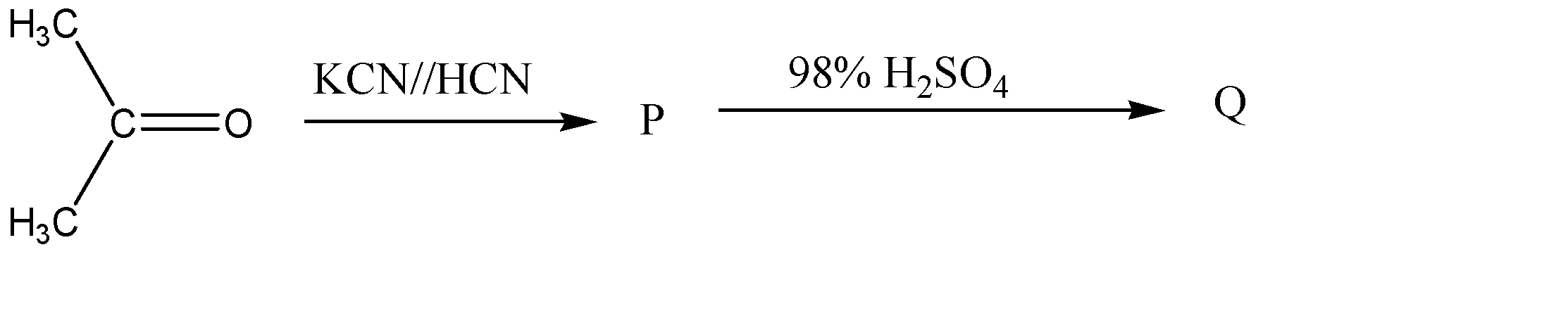
Answer
579.3k+ views
Hint: To solve this question, we need to proceed step by step. We would first determine the product P. then according to the chemical properties of P, we can assess its reaction with the given chemical species to find the final product Q.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
1.The first step of the reaction involves the reaction of propanone with potassium cyanide or hydrogen cyanide. Now, when hydrogen cyanide reacts with carbonyl compounds like aldehyde and ketones, it results in the disruption of the carbon – oxygen double bond, and the hydrogen ion and cyanide ion from the HCN, add themselves on the carbon atom via nucleophilic addition reaction. So, when we react the given reactant, propanone with HCN , the chemical reaction would be represented as follows:
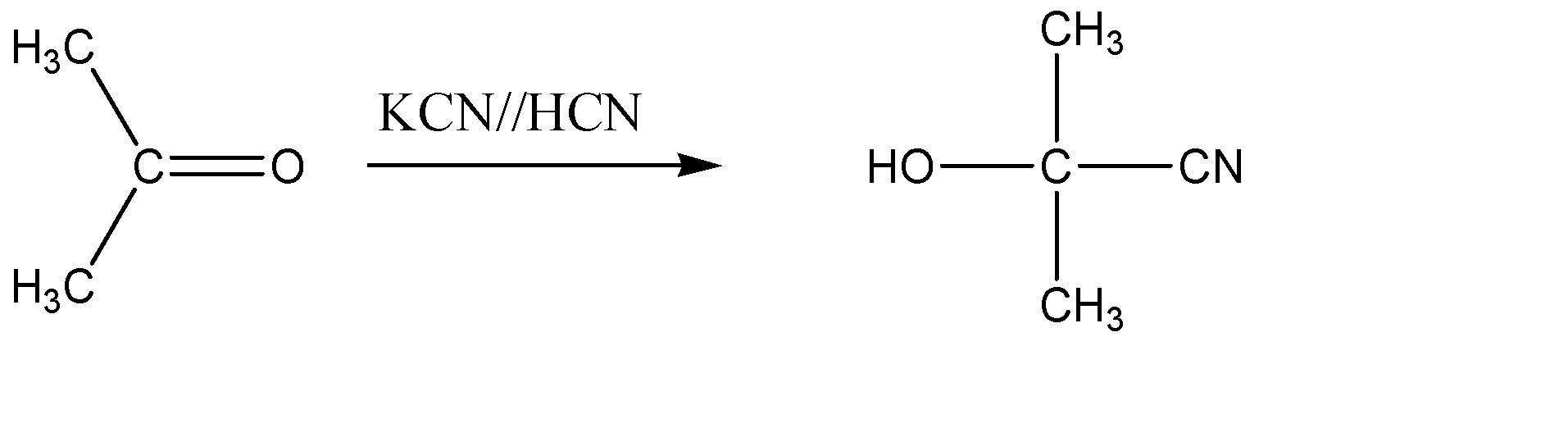
Hence, the product formed is 2 – hydroxy – 2 – methylpropanenitrile.
2.The next step involves the reaction of 2 – hydroxy – 2 – methylpropanenitrile with concentrated sulphuric acid. The sulphuric acid causes the dehydration of the compound. Also, this reaction involves the hydrolyzation of nitrile (CN) group to amide group. This is basically a step – wise process which involves both the dehydration and hydrolysis of 2 – hydroxy – 2 – methylpropanenitrile. The chemical reaction for the same can be given as:
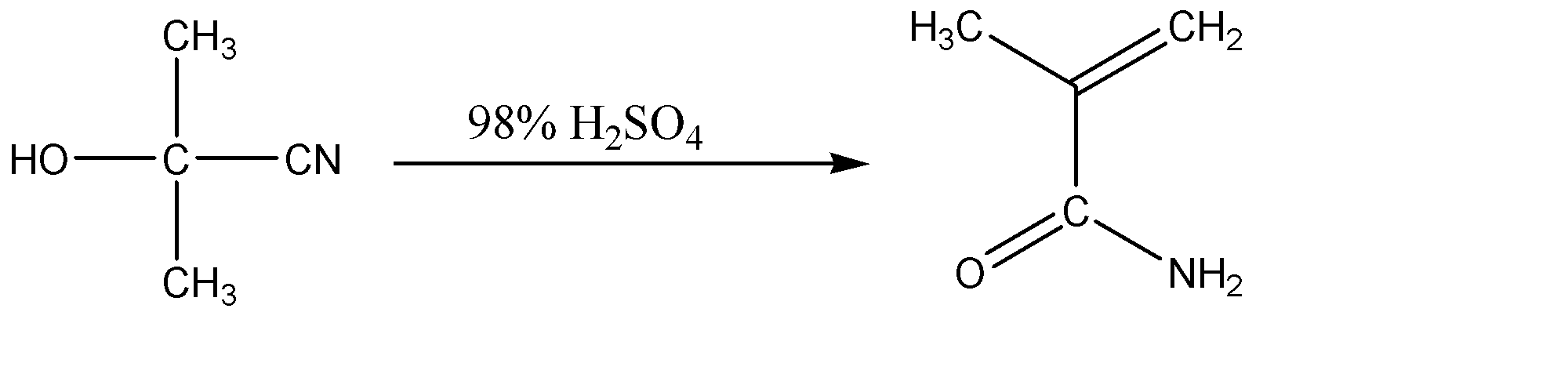
Hence, Q is an amide.
Note: An amide is usually an organic compound that contains a functional group consisting of an acyl group \[\left( {R - C = O} \right)\] linked to a nitrogen atom: The simplest amides are derivatives of ammonia in which one hydrogen atom has been replaced by an acyl group.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
1.The first step of the reaction involves the reaction of propanone with potassium cyanide or hydrogen cyanide. Now, when hydrogen cyanide reacts with carbonyl compounds like aldehyde and ketones, it results in the disruption of the carbon – oxygen double bond, and the hydrogen ion and cyanide ion from the HCN, add themselves on the carbon atom via nucleophilic addition reaction. So, when we react the given reactant, propanone with HCN , the chemical reaction would be represented as follows:

Hence, the product formed is 2 – hydroxy – 2 – methylpropanenitrile.
2.The next step involves the reaction of 2 – hydroxy – 2 – methylpropanenitrile with concentrated sulphuric acid. The sulphuric acid causes the dehydration of the compound. Also, this reaction involves the hydrolyzation of nitrile (CN) group to amide group. This is basically a step – wise process which involves both the dehydration and hydrolysis of 2 – hydroxy – 2 – methylpropanenitrile. The chemical reaction for the same can be given as:

Hence, Q is an amide.
Note: An amide is usually an organic compound that contains a functional group consisting of an acyl group \[\left( {R - C = O} \right)\] linked to a nitrogen atom: The simplest amides are derivatives of ammonia in which one hydrogen atom has been replaced by an acyl group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




