
The structure of the tetrathionate ion is
A.
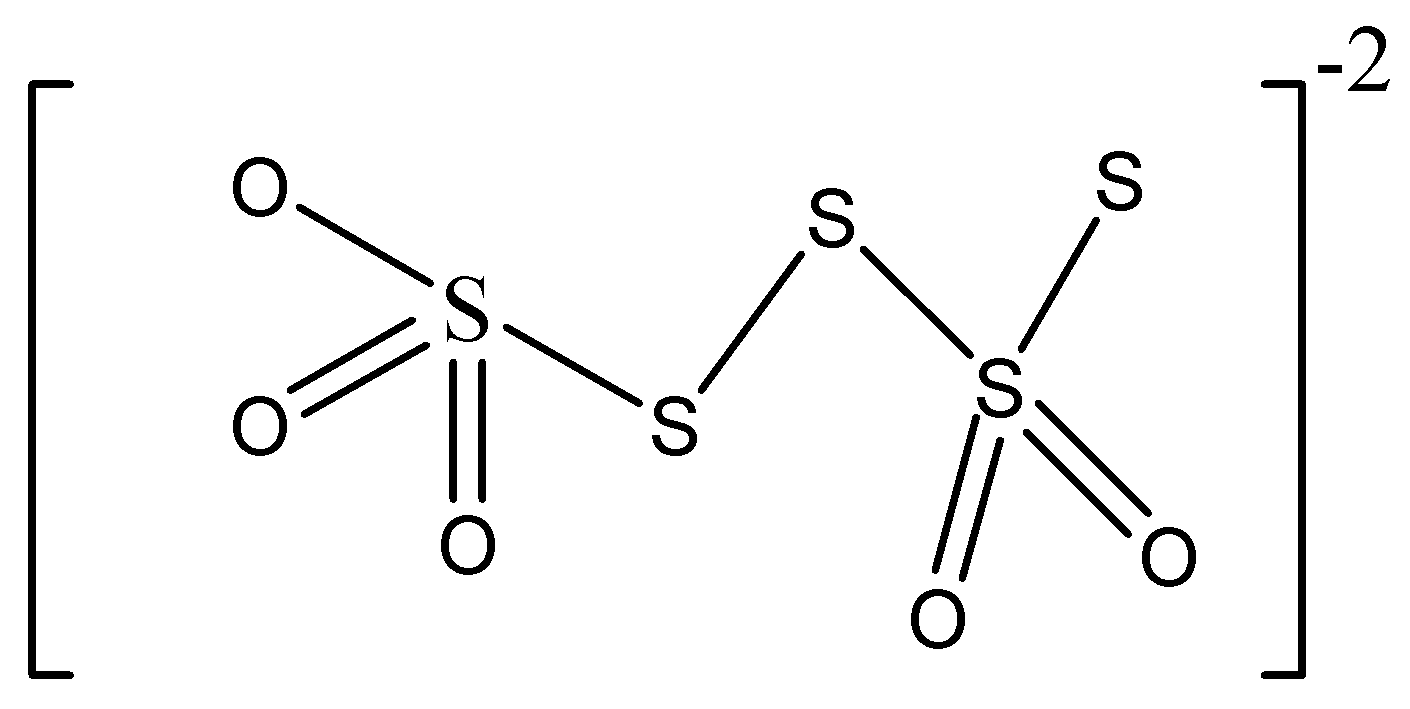
B.
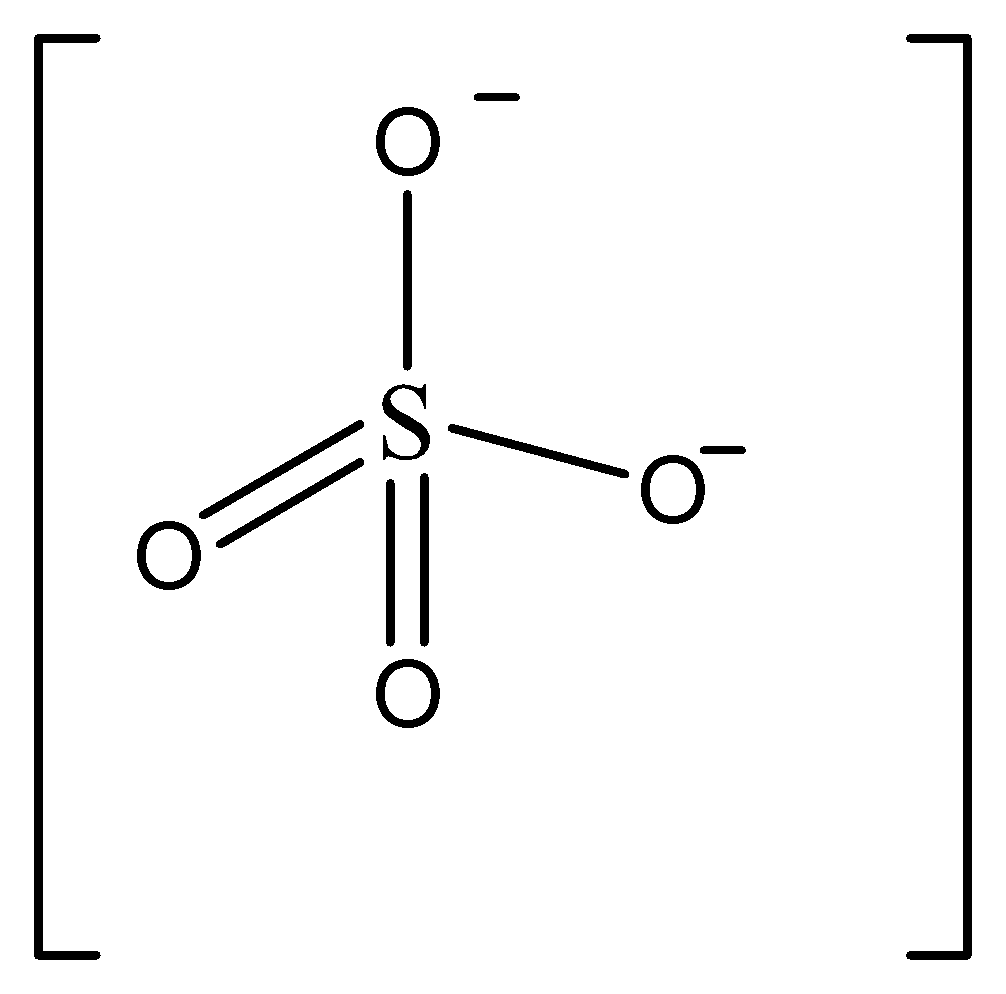
C.
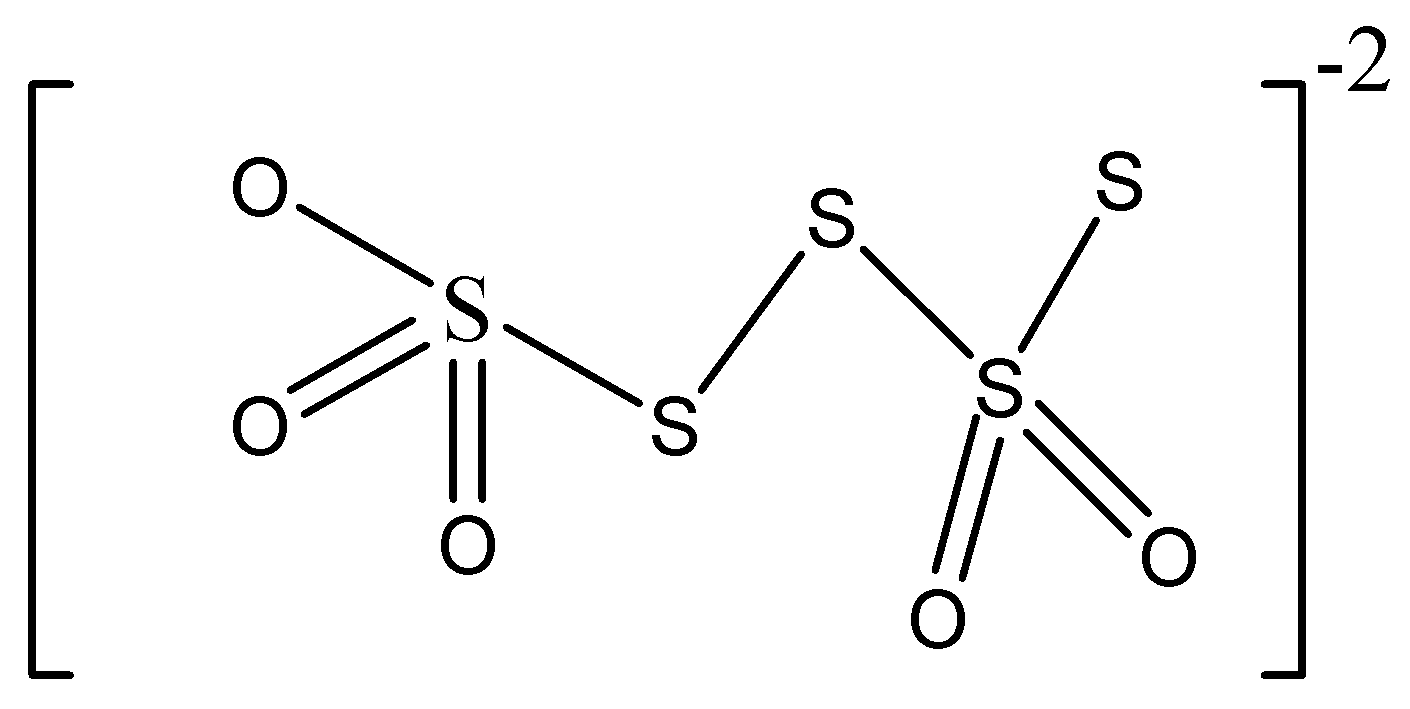
D.
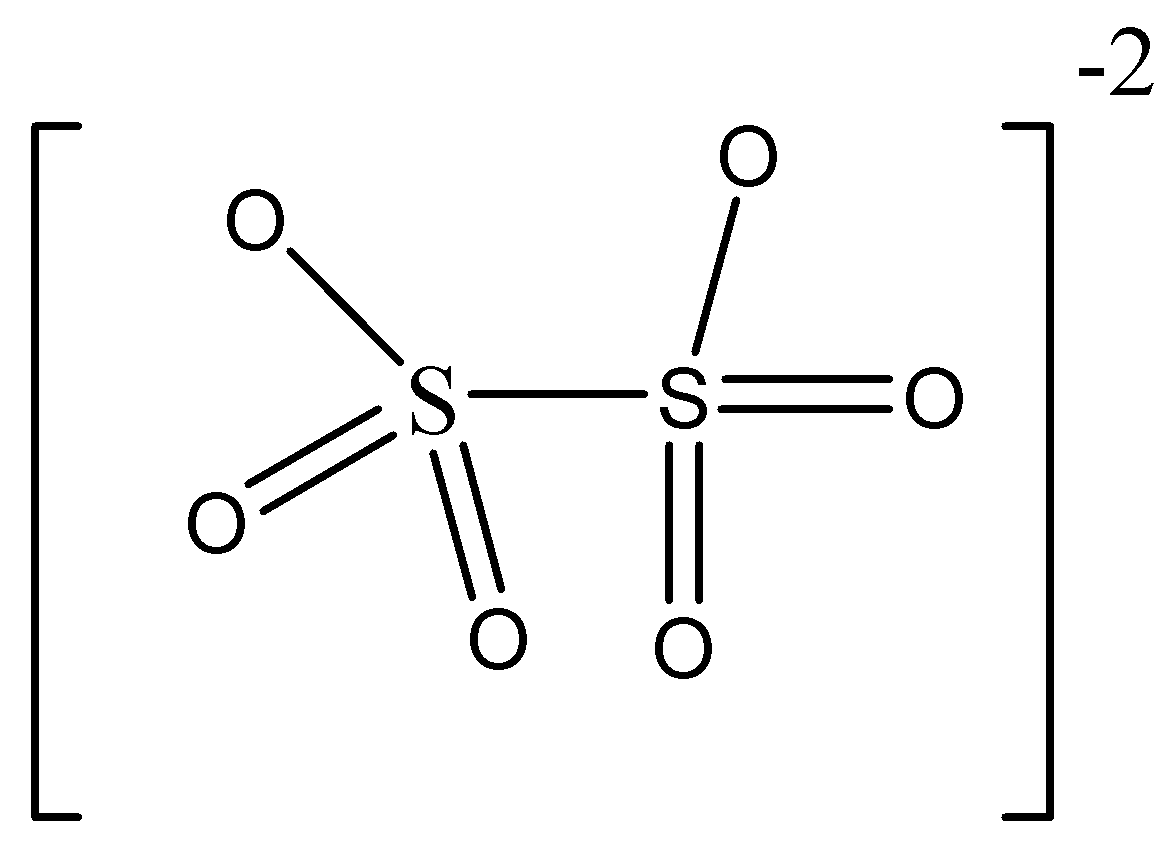




Answer
596.4k+ views
Hint: We must understand that the tetrathionate ion contains four sulphur atoms in a molecule. We can say that the structure mentioned in option C is the structure of tetra-thionate ions.
Complete step by step solution:
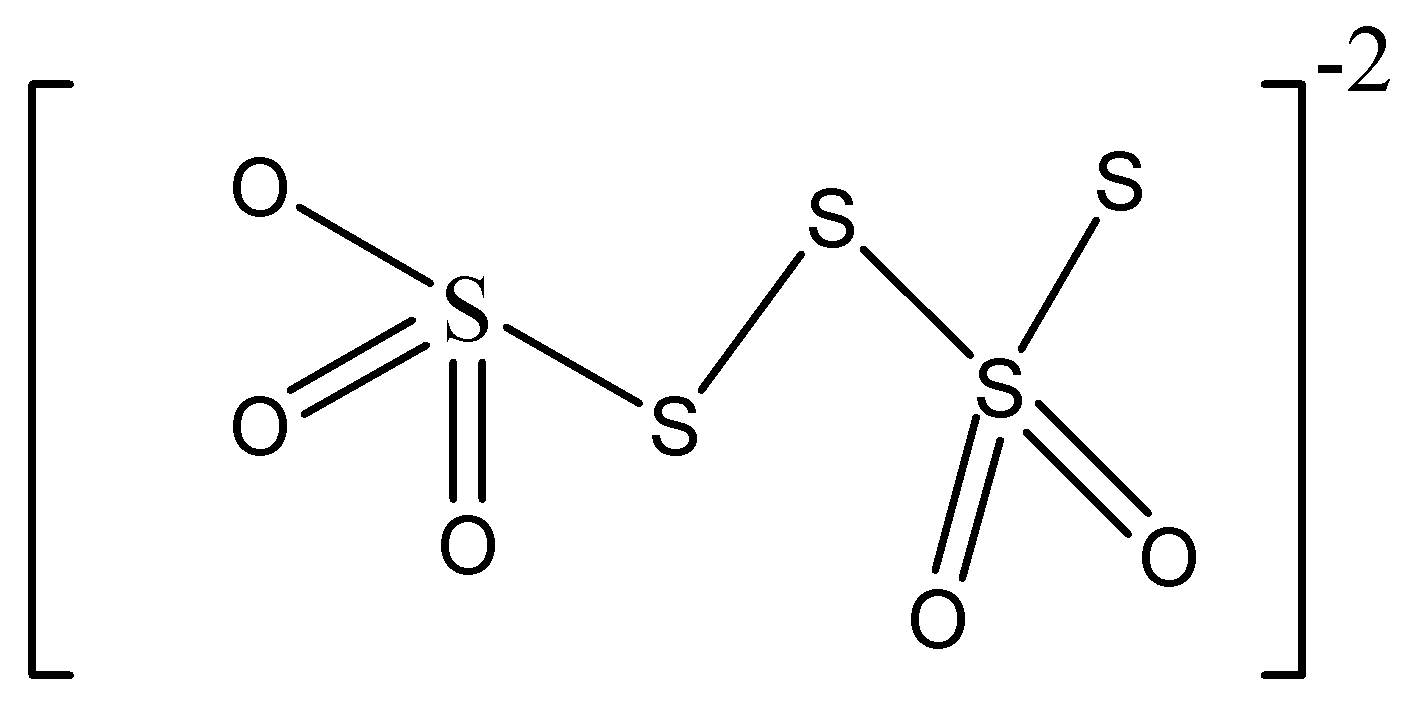
We must know that the chemical formula of the tetra-thionate ion is \[{S_{4}}{O_6}^{2 - }\]
The tetrathionate ion is a sulfur containing oxo-anion that is obtained from the compound called tetra-thionic acid, \[{H_{2}}{S_4}{O_6}.\;\]
Sulphur has various oxidation states, 0, +2, +4, etc. in tetra-thionate ion, two of the sulfur atoms are in oxidation state 0 whereas two are in oxidation state +5.
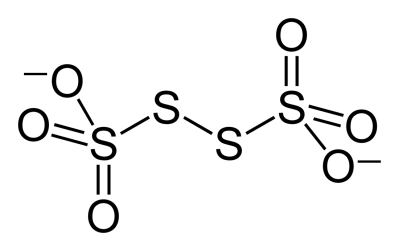
Structure mentioned in option A is polythionate ion that contains more than four sulphur atoms.
So we can understand this is not tetrathionate ion.
Structure mentioned in option B is sulphate ion \[S{O_4}^{2 - }\]
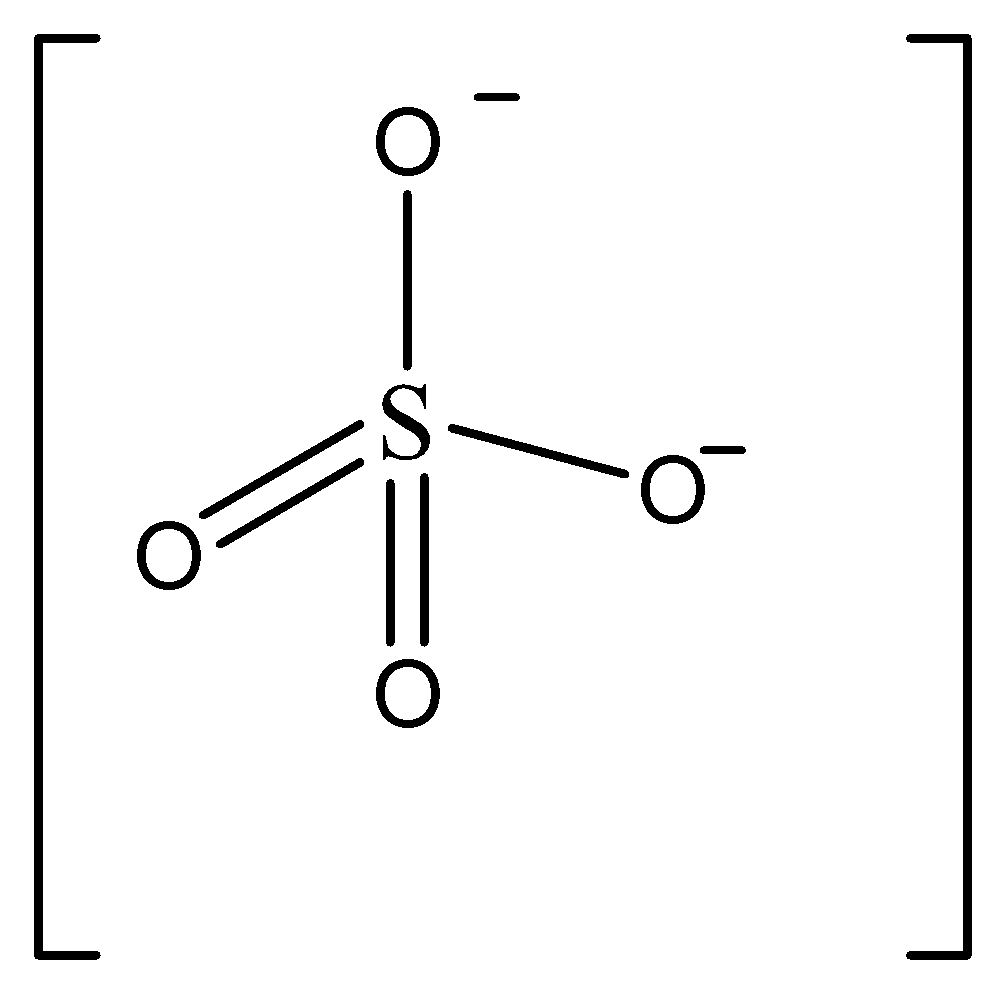
In this structure, we can see there’s only one sulphur atom present.
So we can understand this is not tetrathionate ion.
Structure mentioned in option D is dithionate ion \[{S_2}{O_6}^{2 - }\]
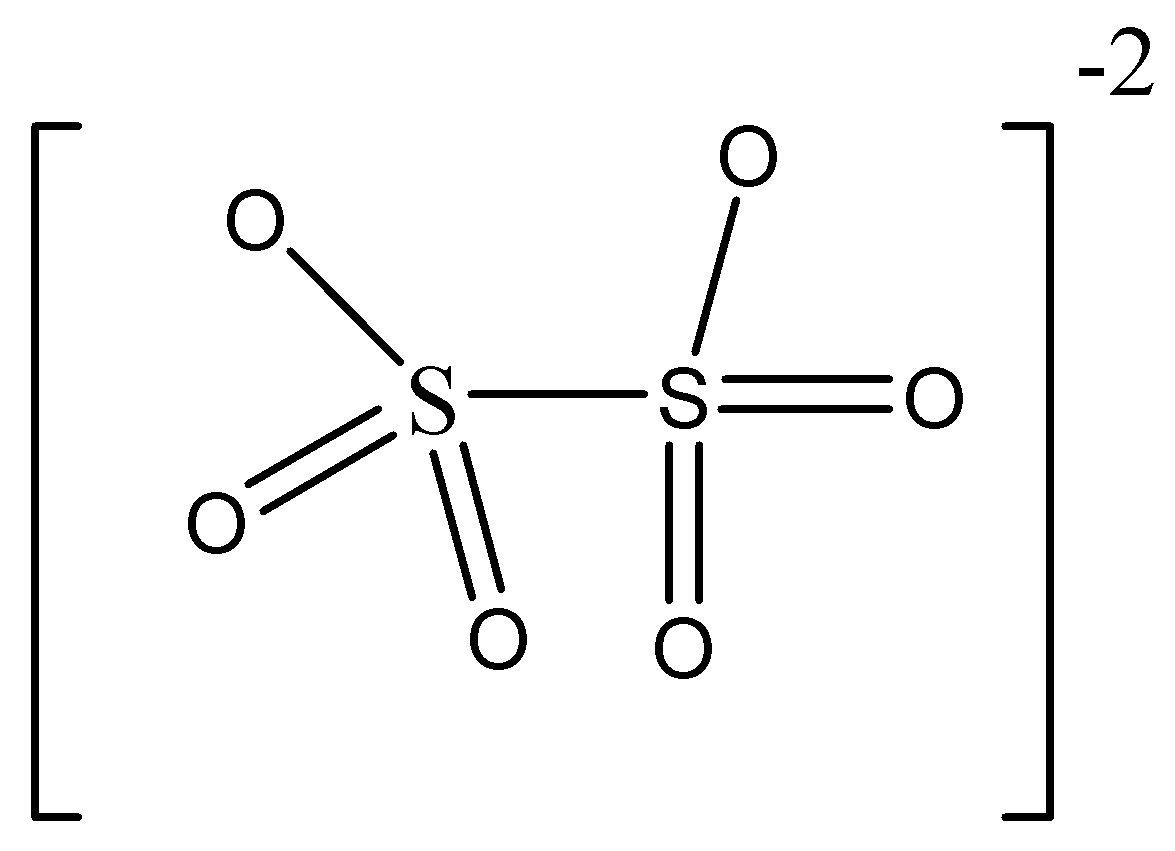
In this structure, we can see there are only two sulphur atoms present. So we can understand this is not tetrathionate ion.
Hence, we can conclude that the option C is the correct option.
Additional information: Tetrathionate can be produced by oxidation of thiosulphate ion \[\left( {{S_2}{O_3}^{2 - }} \right)\] by iodine solution. Major pitting corrosion of carbon steel and stainless steel is due to tetra-thionate ions.
Note: We must understand that the thionate ions have only sulphur and oxygen atoms in their structure. You may get confused with sulphate or such ions that contain sulphur, oxygen as well as R – group or functional group attached to structure.

Complete step by step solution:
We must know that the chemical formula of the tetra-thionate ion is \[{S_{4}}{O_6}^{2 - }\]
The tetrathionate ion is a sulfur containing oxo-anion that is obtained from the compound called tetra-thionic acid, \[{H_{2}}{S_4}{O_6}.\;\]
Sulphur has various oxidation states, 0, +2, +4, etc. in tetra-thionate ion, two of the sulfur atoms are in oxidation state 0 whereas two are in oxidation state +5.
Structure mentioned in option A is polythionate ion that contains more than four sulphur atoms.

So we can understand this is not tetrathionate ion.
Structure mentioned in option B is sulphate ion \[S{O_4}^{2 - }\]

In this structure, we can see there’s only one sulphur atom present.
So we can understand this is not tetrathionate ion.
Structure mentioned in option D is dithionate ion \[{S_2}{O_6}^{2 - }\]

In this structure, we can see there are only two sulphur atoms present. So we can understand this is not tetrathionate ion.
Hence, we can conclude that the option C is the correct option.
Additional information: Tetrathionate can be produced by oxidation of thiosulphate ion \[\left( {{S_2}{O_3}^{2 - }} \right)\] by iodine solution. Major pitting corrosion of carbon steel and stainless steel is due to tetra-thionate ions.
Note: We must understand that the thionate ions have only sulphur and oxygen atoms in their structure. You may get confused with sulphate or such ions that contain sulphur, oxygen as well as R – group or functional group attached to structure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




