
The total number of benzene derivatives having the molecular formula ${C_7}{H_7}Cl$ is:
A. $2$
B. $3$
C. $4$
D. $5$
Answer
573.6k+ views
Hint: Benzene derivatives are compounds with having substituted groups present on a benzene ring. Thus, we can keep away six carbon atoms to form the benzene ring and then check the combinations by which the remaining atoms can be substituted.
Complete step by step answer:
Since we must mandatorily have a benzene ring in each of our products, we can keep away the six carbon atoms needed to form the benzene ring. Thus, we are left with one carbon atom, seven hydrogen atoms and a chlorine atom. The elements which can form substituted groups are therefore, the one carbon and one chlorine atom. Thus, there can be a maximum of two substituted groups on the benzene ring with this molecular formula.
Now let us check how these can be arranged to form different compounds:
Considering the case where there is just one substituent group on the ring: Five hydrogen atoms will be attached to five carbon atoms of the benzene ring. Thus, the remaining two hydrogens along with the chlorine and carbon atoms goes in forming the substituent group. The product we get is known as benzyl chloride:
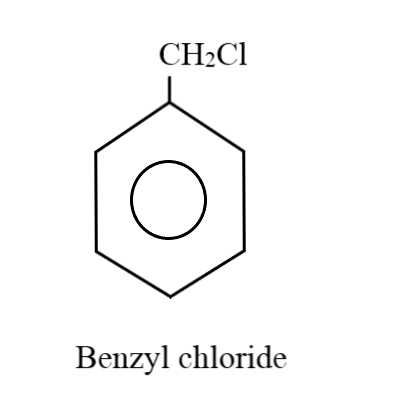
Considering the case where there can be two substituent groups: When there are two substituent groups, only four hydrogens can be normally attached to the carbon atoms in the benzene ring; since the other two carbon atoms will be holding these groups. Placing one carbon atom as a substituent group on one of the corners, the remaining three hydrogen atoms can form a methyl group with this extra carbon atom. The chlorine atom can be at the ortho, meta or para positions, thus, generating three different combinations:
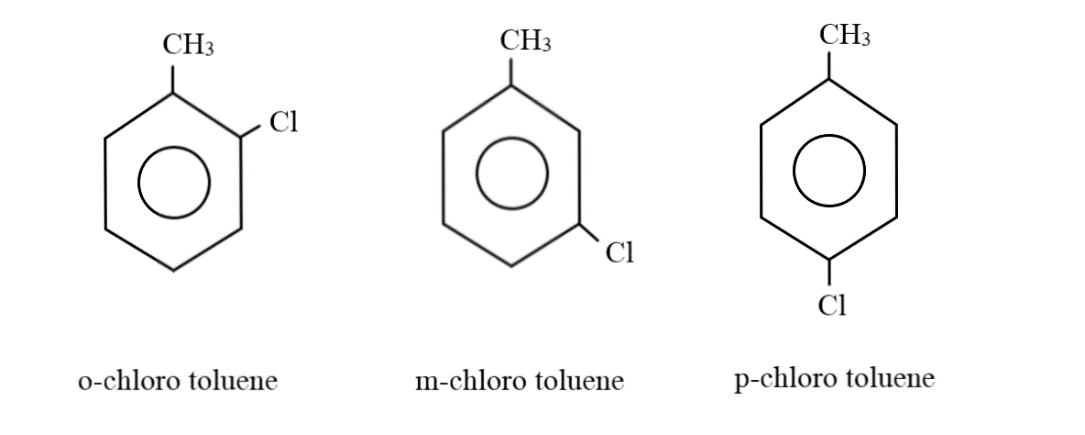
Thus, there are four compounds with the molecular formula ${C_7}{H_7}Cl$, namely Benzyl chloride, o-chlorotoluene, m-chlorotoluene and p-chlorotoluene.
So, the correct answer is Option C.
Note: While considering the elements to be considered as substituent groups, it is to be noted that hydrogen is not taken since it is already a part of the benzene ring and we just need to allocate it to different corners after arranging the substituent groups. Substituted groups on the benzene ring can increase the reactivity of the ring, by acting as either electron donating or electron withdrawing groups.
Complete step by step answer:
Since we must mandatorily have a benzene ring in each of our products, we can keep away the six carbon atoms needed to form the benzene ring. Thus, we are left with one carbon atom, seven hydrogen atoms and a chlorine atom. The elements which can form substituted groups are therefore, the one carbon and one chlorine atom. Thus, there can be a maximum of two substituted groups on the benzene ring with this molecular formula.
Now let us check how these can be arranged to form different compounds:
Considering the case where there is just one substituent group on the ring: Five hydrogen atoms will be attached to five carbon atoms of the benzene ring. Thus, the remaining two hydrogens along with the chlorine and carbon atoms goes in forming the substituent group. The product we get is known as benzyl chloride:

Considering the case where there can be two substituent groups: When there are two substituent groups, only four hydrogens can be normally attached to the carbon atoms in the benzene ring; since the other two carbon atoms will be holding these groups. Placing one carbon atom as a substituent group on one of the corners, the remaining three hydrogen atoms can form a methyl group with this extra carbon atom. The chlorine atom can be at the ortho, meta or para positions, thus, generating three different combinations:

Thus, there are four compounds with the molecular formula ${C_7}{H_7}Cl$, namely Benzyl chloride, o-chlorotoluene, m-chlorotoluene and p-chlorotoluene.
So, the correct answer is Option C.
Note: While considering the elements to be considered as substituent groups, it is to be noted that hydrogen is not taken since it is already a part of the benzene ring and we just need to allocate it to different corners after arranging the substituent groups. Substituted groups on the benzene ring can increase the reactivity of the ring, by acting as either electron donating or electron withdrawing groups.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




