
The total number of cyclic isomers possible for hydrocarbon, ${{\text{C}}_{\text{4}}}{{\text{H}}_6}$ is,
A. $9$
B. $5$
C. $11$
D. $12$
Answer
563.1k+ views
Hint:The molecules having the same molecular formula but a different arrangement of atoms are known as isomers. First, we will determine the degree of unsaturation, so we will know the number of unsaturated bonds. Then we will draw the possible geometries.
Complete solution:
First we will calculate the degree of unsaturation as follows:
${\text{D}}{\text{.U}}\,{\text{ = }}\,{\text{C}} + 1\, - \dfrac{{{\text{no}}{\text{. of monovalent}}\, - \,{\text{no}}{\text{. of}}\,{\text{trivalent}}}}{2}$
On substituting $4$ for C and $6$ for no. of monovalent,
${\text{D}}{\text{.U}}\,{\text{ = }}\,{\text{4}} + 1\, - \dfrac{6}{2}$
\[{\text{D}}{\text{.U}}\,{\text{ = }}\,{\text{4}}\, + 1 - 3\]
\[{\text{D}}{\text{.U}}\,{\text{ = }}2\]
So, the degree of unsaturation is\[2\]. So, we have the possibility of the presence of two double bonds or one triple bond in the structure. We also have the possibility of one ring and one double bond or two rings in the structures.
As we have to find the cyclic isomers only, we will find the structures having one ring with one double bond or two rings.
Structure with two rings:
The possible geometry of four carbon atoms with two ring is shown as follows:
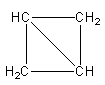
The above structure has two three-membered rings and ${{\text{C}}_{\text{4}}}{{\text{H}}_{\text{6}}}$ molecular formula.
Structure with one ring and one double bond:
We can draw the structure having one four-membered ring and one double bond as follows:
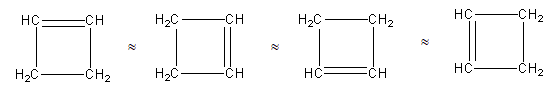
Above all four structures are the same as all four have the same chemical formula.
The highest possible ring size with four carbon is of a four-membered ring which we had drawn already, so we will try with three-membered cyclic structures.
The possible geometry of four carbon atoms with one ring and one double bond is shown as follows:
We can draw a three-membered cyclic structure with one double bond and one methyl group.
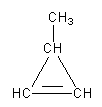
The second possibility is that we can draw the double bond outside of the ring as follows:
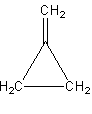
The third possibility is that we can shift the position in the ring as follows:
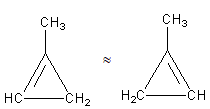
The above structures are the same as both have the same chemical formula.
So, the total number of cyclic isomers possible for hydrocarbon, ${{\text{C}}_{\text{4}}}{{\text{H}}_6}$ is, $5$.
Therefore, the correct answer is (C).
Note: Isomers have the same molecular formula but different chemical formula. A molecular formula shows the total number of an atom in the compound. The chemical formula shows the different group of atoms of a molecule. Here, ${{\text{C}}_{\text{4}}}{{\text{H}}_6}$is the molecular formula but the ${\text{C}}{{\text{H}}_{\text{3}}}{\text{CCHC}}{{\text{H}}_{\text{2}}}$ is the chemical formula. By the general formula of hydrocarbon, we will decide that the given molecular formula is representing the alkane, alkene, or alkyne. The general formula of the alkyne is, ${{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n - 2}}}}$ .Where, n is the number of carbon atoms. So, ${{\text{C}}_{\text{4}}}{{\text{H}}_6}$ is alkyne. So, we can also draw the non-cyclic isomers having a triple bond.
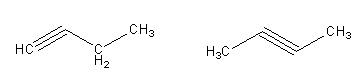
Complete solution:
First we will calculate the degree of unsaturation as follows:
${\text{D}}{\text{.U}}\,{\text{ = }}\,{\text{C}} + 1\, - \dfrac{{{\text{no}}{\text{. of monovalent}}\, - \,{\text{no}}{\text{. of}}\,{\text{trivalent}}}}{2}$
On substituting $4$ for C and $6$ for no. of monovalent,
${\text{D}}{\text{.U}}\,{\text{ = }}\,{\text{4}} + 1\, - \dfrac{6}{2}$
\[{\text{D}}{\text{.U}}\,{\text{ = }}\,{\text{4}}\, + 1 - 3\]
\[{\text{D}}{\text{.U}}\,{\text{ = }}2\]
So, the degree of unsaturation is\[2\]. So, we have the possibility of the presence of two double bonds or one triple bond in the structure. We also have the possibility of one ring and one double bond or two rings in the structures.
As we have to find the cyclic isomers only, we will find the structures having one ring with one double bond or two rings.
Structure with two rings:
The possible geometry of four carbon atoms with two ring is shown as follows:

The above structure has two three-membered rings and ${{\text{C}}_{\text{4}}}{{\text{H}}_{\text{6}}}$ molecular formula.
Structure with one ring and one double bond:
We can draw the structure having one four-membered ring and one double bond as follows:

Above all four structures are the same as all four have the same chemical formula.
The highest possible ring size with four carbon is of a four-membered ring which we had drawn already, so we will try with three-membered cyclic structures.
The possible geometry of four carbon atoms with one ring and one double bond is shown as follows:
We can draw a three-membered cyclic structure with one double bond and one methyl group.

The second possibility is that we can draw the double bond outside of the ring as follows:

The third possibility is that we can shift the position in the ring as follows:

The above structures are the same as both have the same chemical formula.
So, the total number of cyclic isomers possible for hydrocarbon, ${{\text{C}}_{\text{4}}}{{\text{H}}_6}$ is, $5$.
Therefore, the correct answer is (C).
Note: Isomers have the same molecular formula but different chemical formula. A molecular formula shows the total number of an atom in the compound. The chemical formula shows the different group of atoms of a molecule. Here, ${{\text{C}}_{\text{4}}}{{\text{H}}_6}$is the molecular formula but the ${\text{C}}{{\text{H}}_{\text{3}}}{\text{CCHC}}{{\text{H}}_{\text{2}}}$ is the chemical formula. By the general formula of hydrocarbon, we will decide that the given molecular formula is representing the alkane, alkene, or alkyne. The general formula of the alkyne is, ${{\text{C}}_{\text{n}}}{{\text{H}}_{{\text{2n - 2}}}}$ .Where, n is the number of carbon atoms. So, ${{\text{C}}_{\text{4}}}{{\text{H}}_6}$ is alkyne. So, we can also draw the non-cyclic isomers having a triple bond.
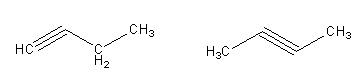
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




