
The total number of electrons in one molecule of carbon dioxide is (A)
A. 22
B. 44
C. 66
D. 88
Answer
592.5k+ views
Hint: Valency theory & Chemical bonding model can tell about the number of electrons in one molecule of carbon dioxide. Valence bond theory considers that the overlapping atomic orbitals of the participating atoms form a chemical bond between the atoms and because of this overlapping, it is most probable that electrons should be available in the bond region. Valence bond theory views bonds as weakly coupled orbitals or small overlap.
Complete answer:
Carbon dioxide has the formula $C{{O}_{2}}$ and at the center of this linear molecule is a carbon atom joined by two pairs of double-bonds to the oxygen atoms, that is O=C=O. At room temperature carbon dioxide is a colorless gas which has a slightly sweet smell. Carbon dioxide $=C{{O}_{2}}$
Lewis structure
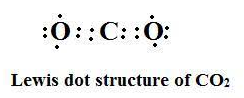
There are no loss/ gain of electrons during formation of $C{{O}_{2}}$
Hence from electronic configurations
$C:1{{s}^{2}}2{{s}^{2}}2{{p}^{2}}:6{{e}^{-}}$
$O:1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}:8{{e}^{-}}$
electron in one carbon atom is 6 and electron present in two of the oxygen atom and hence total number of electrons $=6+2(8)=22$
$(C)+2(O)$
So, the correct answer is “Option A”.
Note: Lewis structures which are also known as Lewis dot diagrams or Lewis electron dot structures, are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.Lewis structures were only used to determine the type of bond and loss/gain of elections during the formation of compound in older times. Now, there are new and advanced theories.
Complete answer:
Carbon dioxide has the formula $C{{O}_{2}}$ and at the center of this linear molecule is a carbon atom joined by two pairs of double-bonds to the oxygen atoms, that is O=C=O. At room temperature carbon dioxide is a colorless gas which has a slightly sweet smell. Carbon dioxide $=C{{O}_{2}}$
Lewis structure
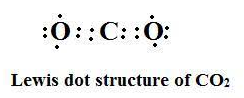
There are no loss/ gain of electrons during formation of $C{{O}_{2}}$
Hence from electronic configurations
$C:1{{s}^{2}}2{{s}^{2}}2{{p}^{2}}:6{{e}^{-}}$
$O:1{{s}^{2}}2{{s}^{2}}2{{p}^{4}}:8{{e}^{-}}$
electron in one carbon atom is 6 and electron present in two of the oxygen atom and hence total number of electrons $=6+2(8)=22$
$(C)+2(O)$
So, the correct answer is “Option A”.
Note: Lewis structures which are also known as Lewis dot diagrams or Lewis electron dot structures, are diagrams that show the bonding between atoms of a molecule and the lone pairs of electrons that may exist in the molecule.Lewis structures were only used to determine the type of bond and loss/gain of elections during the formation of compound in older times. Now, there are new and advanced theories.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




