
The type of hybridization of boron in diborane is?
Answer
559.5k+ views
Hint: Boron is an element present in group $15$ of the periodic table. It has three valence electrons in its outermost shell thus it is capable of making bonds accordingly. The formula of Diborane is${B_2}{H_6}$.
We know that boron has three electrons in its outermost shell. Thus with the hydrogen atoms, it must make bonds as per the number of valence electrons which means if three valence electrons are there thus three bonds must be made. If there were three bonds made between Boron and Hydrogen atoms, then the hybridisation would have been $s{p_2}$.
Complete step by step answer:
But in the compound${B_2}{H_6}$, this is not the case. The structure is made as follows:
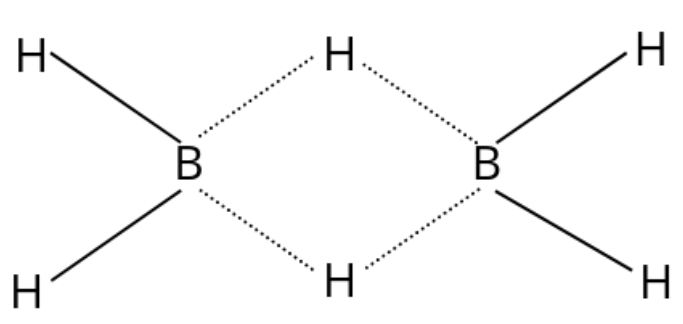
Now in this, one boron forms four bonds. By each boron atom, three bonds are made with hydrogen atoms which are individual and one bond is made with the hydrogen atom of the other boron. This causes the presence of $12$ valence electrons to be present in the valence shell of each boron atom.
The hybridisation of the boron atom now becomes $s{p_3}$ due to the four bonds formed. In the structure, there are two hydrogens on the left which are known as terminal hydrogens. The terminal hydrogens are in different environments from the two hydrogen atoms on the right other which are called bridging atoms. The bridging atoms are present on the right side.
Thus the hybridisation of each boron atom is $s{p_3}$ with the tetrahedral geometry.
Note: There is a difference in the plane of the atoms which are the two bridging hydrogen atoms, the two boron atoms and the four terminal hydrogen atoms. The four terminal hydrogen atoms and two boron atoms are present in an identical plan. The two bridging hydrogen atoms which are one above and the other one is below and two boron atoms are present in a plane perpendicular to the other plane.
We know that boron has three electrons in its outermost shell. Thus with the hydrogen atoms, it must make bonds as per the number of valence electrons which means if three valence electrons are there thus three bonds must be made. If there were three bonds made between Boron and Hydrogen atoms, then the hybridisation would have been $s{p_2}$.
Complete step by step answer:
But in the compound${B_2}{H_6}$, this is not the case. The structure is made as follows:

Now in this, one boron forms four bonds. By each boron atom, three bonds are made with hydrogen atoms which are individual and one bond is made with the hydrogen atom of the other boron. This causes the presence of $12$ valence electrons to be present in the valence shell of each boron atom.
The hybridisation of the boron atom now becomes $s{p_3}$ due to the four bonds formed. In the structure, there are two hydrogens on the left which are known as terminal hydrogens. The terminal hydrogens are in different environments from the two hydrogen atoms on the right other which are called bridging atoms. The bridging atoms are present on the right side.
Thus the hybridisation of each boron atom is $s{p_3}$ with the tetrahedral geometry.
Note: There is a difference in the plane of the atoms which are the two bridging hydrogen atoms, the two boron atoms and the four terminal hydrogen atoms. The four terminal hydrogen atoms and two boron atoms are present in an identical plan. The two bridging hydrogen atoms which are one above and the other one is below and two boron atoms are present in a plane perpendicular to the other plane.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




