
What is the total number of possible isomeric trimethylbenzene?
Answer
513.3k+ views
Hint: Isomers in chemistry are molecules or polyatomic ions that have the same molecular formula — that is, the same number of atoms of each element — but different atomic configurations in space. The existence or potential of isomers is referred to as isomerism. The chemical and physical properties of isomers are not necessarily the same.
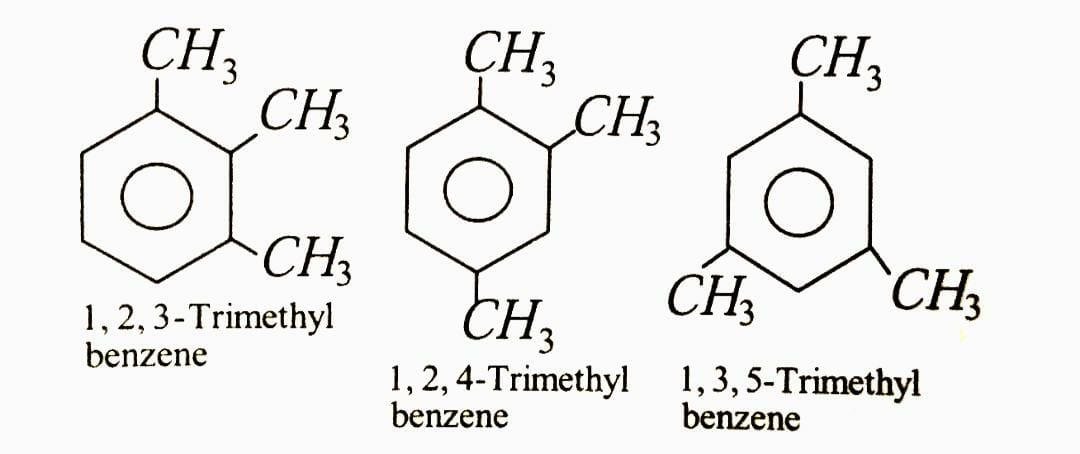
Complete answer: The total number of possible isomeric trimethylbenzene is 3
The organic compound \[1,2,3 - trimethylbenzene\] has the chemical formula \[{C_6}{H_3}{\left( {C{H_3}} \right)_3}\] . It's an aromatic hydrocarbon, which means it's combustible and colourless. It's almost insoluble in water, but it's soluble in organic solvents. Coal tar and petroleum both contain it.
The organic compound \[1,2,4 - trimethylbenzene\] , often known as pseudocumene, has the chemical formula \[{C_6}{H_3}{\left( {C{H_3}} \right)_3}\] .
Mesitylene, also known as \[1,3,5 - trimethylbenzene\] , is a benzene derivative containing three methyl substituents symmetrically positioned around the ring. \[1,2,4 - trimethylbenzene\] and \[1,2,3 - trimethylbenzene\] are the other two isomeric trimethylbenzenes.
Note:
\[1,2,4 - trimethylbenzene\] is used as a solvent, as a paint and lacquer thinner, in the manufacture of colours, and in the production of prescription medications, in addition to being part of the chemical used as a gasoline additive. Breathing high quantities of \[1,2,4 - trimethylbenzene\] over short periods of time has an effect on the nervous system, resulting in headaches, fatigue, sleepiness, or dizziness. Coughing, wheezing, and/or shortness of breath are caused by inhaling \[1,2,4 - trimethylbenzene\] vapour, which irritates the nose, throat, and lungs. Muscle control issues, anxiety, and bewilderment are other indications of exposure. The effects \[1,2,4 - trimethylbenzene\] might appear immediately or shortly after exposure.
Complete answer: The total number of possible isomeric trimethylbenzene is 3
The organic compound \[1,2,3 - trimethylbenzene\] has the chemical formula \[{C_6}{H_3}{\left( {C{H_3}} \right)_3}\] . It's an aromatic hydrocarbon, which means it's combustible and colourless. It's almost insoluble in water, but it's soluble in organic solvents. Coal tar and petroleum both contain it.
The organic compound \[1,2,4 - trimethylbenzene\] , often known as pseudocumene, has the chemical formula \[{C_6}{H_3}{\left( {C{H_3}} \right)_3}\] .
Mesitylene, also known as \[1,3,5 - trimethylbenzene\] , is a benzene derivative containing three methyl substituents symmetrically positioned around the ring. \[1,2,4 - trimethylbenzene\] and \[1,2,3 - trimethylbenzene\] are the other two isomeric trimethylbenzenes.

Note:
\[1,2,4 - trimethylbenzene\] is used as a solvent, as a paint and lacquer thinner, in the manufacture of colours, and in the production of prescription medications, in addition to being part of the chemical used as a gasoline additive. Breathing high quantities of \[1,2,4 - trimethylbenzene\] over short periods of time has an effect on the nervous system, resulting in headaches, fatigue, sleepiness, or dizziness. Coughing, wheezing, and/or shortness of breath are caused by inhaling \[1,2,4 - trimethylbenzene\] vapour, which irritates the nose, throat, and lungs. Muscle control issues, anxiety, and bewilderment are other indications of exposure. The effects \[1,2,4 - trimethylbenzene\] might appear immediately or shortly after exposure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




