
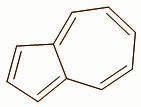
Unlike most hydrocarbons, azulene $\left( {{{\text{C}}_{{\text{10}}}}{{\text{H}}_{\text{8}}}} \right)$ is highly coloured (deep blue), although its isomer, naphthalene does not have significant zwitter-ionic character, azulene does.
1.Draw a resonance structure of azulene in which a five membered ring is anionic and the seven membered ring cationic.
2.Can azulene be considered aromatic?

Answer
573k+ views
Hint: To solve this question, you must recall the Huckel rule for determining the aromaticity of a compound. You must also recall the rules for writing the possible resonance structures of a molecule. Resonance structures represent the delocalization of pi electrons.
Complete step by step solution:
Resonance occurs when there is a conjugation of pi bonds. In other words, an alternate double single double bond arrangement. Such a system is known as a conjugate system. It also takes place when there is a double bond along with a group present in the carbon chain having a lone pair.
We can draw the resonance structure of azulene with an anionic five membered ring and cationic seven membered ring as:

For a compound to be aromatic, it must follow the Huckel rule.
According to this rule, the conditions for a compound to be aromatic are:
1.It should be cyclical
2.It should be planar
3.It should possess complete conjugation
4.It must have $4{\text{n}} + 2$ pi electrons, where n is a whole number
We know that azulene is cyclic, planar as well as has complete conjugation.
There are five double bonds that are in complete conjugation. So the number of delocalized pi electrons is 10.
Thus, we can say that azulene is an aromatic compound.
Note: When a molecule or ion can be represented by two or more structures which have the same arrangement of atomic nuclei but differ in the distribution of electrons. This phenomenon is called resonance. The various structures involved are known as resonating structures. The resonating structures do not represent all the properties of the molecule or ion. The actual structure of the molecule or ion is the resonance hybrid of all the resonating structures.
Complete step by step solution:
Resonance occurs when there is a conjugation of pi bonds. In other words, an alternate double single double bond arrangement. Such a system is known as a conjugate system. It also takes place when there is a double bond along with a group present in the carbon chain having a lone pair.
We can draw the resonance structure of azulene with an anionic five membered ring and cationic seven membered ring as:

For a compound to be aromatic, it must follow the Huckel rule.
According to this rule, the conditions for a compound to be aromatic are:
1.It should be cyclical
2.It should be planar
3.It should possess complete conjugation
4.It must have $4{\text{n}} + 2$ pi electrons, where n is a whole number
We know that azulene is cyclic, planar as well as has complete conjugation.
There are five double bonds that are in complete conjugation. So the number of delocalized pi electrons is 10.
Thus, we can say that azulene is an aromatic compound.
Note: When a molecule or ion can be represented by two or more structures which have the same arrangement of atomic nuclei but differ in the distribution of electrons. This phenomenon is called resonance. The various structures involved are known as resonating structures. The resonating structures do not represent all the properties of the molecule or ion. The actual structure of the molecule or ion is the resonance hybrid of all the resonating structures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




