
How do I use Lewis structures to determine the oxidation numbers of \[N\] in compounds like \[{N_2}O\], \[NO\] , \[{N_2}{O_3}\], and \[{N_2}{O_5}\]?
Answer
558k+ views
Hint: To deal with this question we need to have an idea about Lewis structures especially on certain rules to be followed during using lewis structures to determine the oxidation number. Actually what we are going to do here is just counting the valence electrons around nitrogen according to those rules and after that an oxidation number is assigned. Actually oxidation number is the difference between valence electron in isolated atom and valence electron in bound atom.
Complete step by step answer:
Before proceeding to the calculation of oxidation number we have to look into certain rules to be followed during this as mentioned in the hint. The rules are as follows,
\[1.\] Lone pair of electrons should belong to the atom on which they reside.
\[2.\] Bonding pair electrons or shared electrons should be shared equally between identical atoms.
\[3.\] Shared electrons should be between different atoms belonging to more electronegative atoms.
\[4.\] Oxidation number is the difference between valence electron in isolated atom and valence electron in bound atom.
Next our aim is to find the oxidation number. Before that let us note the valence electrons in isolated atoms of nitrogen \[(VE)\] which will be common in all the compounds asked in question as the isolated atom is nitrogen in each of the compounds. So no need to find it separately in each compound.
So, \[VE\]\[ = 5\] (isolated nitrogen atom has five valence electrons)
First of all we have to find the lewis structure of dinitrogen monoxide \[({N_2}O)\] .
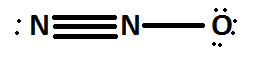
Here there are two nitrogen atoms hence oxidation number should be found separately on each nitrogen atom and at last we will take the average of both to get the final oxidation number.First of all let us look at the oxidation number of the left hand nitrogen atom.
\[LP = 2\]; \[BE = 3\]
\[ON = VE - LP - BE\]
\[ = 5 - 2 - 3 = 0\]
Next we have to look into the central nitrogen atom.
\[LP = 0;BE = 3\]
\[ON = 5 - 0 - 3 = + 2\]
Average should be taken as each nitrogen atom possesses different formal charges.
Average oxidation number \[ = \dfrac{{0 + 2}}{2} = + \dfrac{1}{2}\]
Next we can take the case of nitrogen monoxide \[(NO)\] .
The Lewis structure of \[NO\] is
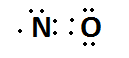
Here , \[LP = 3;BE = 0\]
\[ON = VE - LP - BE\]
\[ON = 5 - 3 - 0 = + 2\]
Next we shall take the case of dinitrogen trioxide \[{N_2}{O_3}\] .
The Lewis structure of \[{N_2}{O_3}\] is
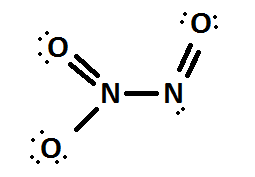
Here also there are two nitrogen atoms so that we should separately calculate the oxidation number on each and will take the average at last as did dinitrogen monoxide.
First of all let us look at the left hand nitrogen atom.
Here, \[LP = 0;BE = 1\]
\[ON = VE - LP - BE\]
\[ON = 5 - 0 - 1 = + 4\]
Next let us find the central nitrogen atom.
Here, \[LP = 2;BE = 1\]
\[ON = VE - LP - BE\]
\[ON = 5 - 2 - 1 = + 2\]
Average formal charge \[ = \dfrac{{4 + 2}}{2} = + 3\]
Next let us take the case of dinitrogen tetroxide \[({N_2}{O_4})\]
The Lewis structure of \[{N_2}{O_4}\] is
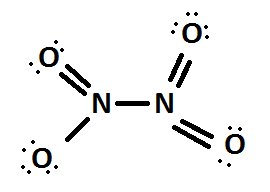
Here also two nitrogen atoms. So let us find the oxidation number as in the same method according to the above two compounds on which we had already done.
On left hand nitrogen atom,
\[LP = 0;BE = 1\]
\[ON = VE - LP - BE\]
\[ON = 5 - 0 - 1 = + 4\]
On right hand nitrogen atom,
\[LP = 0;BE = 1\]
\[ON = VE - LP - BE\]
\[ON = 5 - 0 - 1 = + 4\]
Here both sides are having the same formal charge therefore the oxidation number is definitely \[ + 4\] if we take the average also.
Next we have to take the case of the last compound given which is dinitrogen pentoxide \[({N_2}{O_5})\].
The Lewis structure of \[{N_2}{O_5}\] is
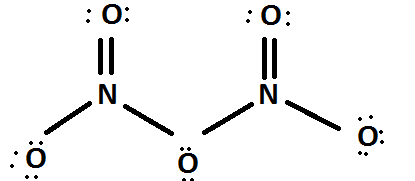
Here also we can know that there are two nitrogen atoms. So , let us follow the same steps which we had already done above in three compounds having two nitrogen atoms.
On left hand side, nitrogen atom it is having as follows,
\[LP = 0;BE = 0\]
\[ON = VE - LP - BE\]
\[ON = 5 - 0 - 0 = + 5\]
On right hand side , nitrogen atom is having as follows,
\[LP = 0;BE = 0\]
\[ON = VE - LP - BE\]
\[ON = 5 - 0 - 0 = + 5\]
Here both sides are having the same formal charge therefore the oxidation number is definitely \[ + 5\] if we take the average also.
In this way Lewis structure is used to to determine the oxidation numbers of \[N\] in compounds like \[{N_2}O\], \[NO\] , \[{N_2}{O_3}\], and \[{N_2}{O_5}\] .
Note: To deal with this type of question first of all we should know about the lewis structure of the compounds because then only we can easily find out the lone pairs and valence electrons in bound atoms which in turn helps to determine the oxidation number. Actually one main thing we have to know is that there is no matter with the bonding which we decide on Lewis dot structures, because in sense we have to destroy that to figure out oxidation states. Also , it makes us always revert back to pretend about ionic structures where electrons are not shared but only transferred to more electronegative atoms. We should follow the rules mentioned in the solution part while determining oxidation number using lewis dot structures so as to find without error.
Complete step by step answer:
Before proceeding to the calculation of oxidation number we have to look into certain rules to be followed during this as mentioned in the hint. The rules are as follows,
\[1.\] Lone pair of electrons should belong to the atom on which they reside.
\[2.\] Bonding pair electrons or shared electrons should be shared equally between identical atoms.
\[3.\] Shared electrons should be between different atoms belonging to more electronegative atoms.
\[4.\] Oxidation number is the difference between valence electron in isolated atom and valence electron in bound atom.
Next our aim is to find the oxidation number. Before that let us note the valence electrons in isolated atoms of nitrogen \[(VE)\] which will be common in all the compounds asked in question as the isolated atom is nitrogen in each of the compounds. So no need to find it separately in each compound.
So, \[VE\]\[ = 5\] (isolated nitrogen atom has five valence electrons)
First of all we have to find the lewis structure of dinitrogen monoxide \[({N_2}O)\] .
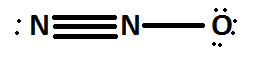
Here there are two nitrogen atoms hence oxidation number should be found separately on each nitrogen atom and at last we will take the average of both to get the final oxidation number.First of all let us look at the oxidation number of the left hand nitrogen atom.
\[LP = 2\]; \[BE = 3\]
\[ON = VE - LP - BE\]
\[ = 5 - 2 - 3 = 0\]
Next we have to look into the central nitrogen atom.
\[LP = 0;BE = 3\]
\[ON = 5 - 0 - 3 = + 2\]
Average should be taken as each nitrogen atom possesses different formal charges.
Average oxidation number \[ = \dfrac{{0 + 2}}{2} = + \dfrac{1}{2}\]
Next we can take the case of nitrogen monoxide \[(NO)\] .
The Lewis structure of \[NO\] is
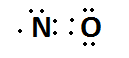
Here , \[LP = 3;BE = 0\]
\[ON = VE - LP - BE\]
\[ON = 5 - 3 - 0 = + 2\]
Next we shall take the case of dinitrogen trioxide \[{N_2}{O_3}\] .
The Lewis structure of \[{N_2}{O_3}\] is

Here also there are two nitrogen atoms so that we should separately calculate the oxidation number on each and will take the average at last as did dinitrogen monoxide.
First of all let us look at the left hand nitrogen atom.
Here, \[LP = 0;BE = 1\]
\[ON = VE - LP - BE\]
\[ON = 5 - 0 - 1 = + 4\]
Next let us find the central nitrogen atom.
Here, \[LP = 2;BE = 1\]
\[ON = VE - LP - BE\]
\[ON = 5 - 2 - 1 = + 2\]
Average formal charge \[ = \dfrac{{4 + 2}}{2} = + 3\]
Next let us take the case of dinitrogen tetroxide \[({N_2}{O_4})\]
The Lewis structure of \[{N_2}{O_4}\] is

Here also two nitrogen atoms. So let us find the oxidation number as in the same method according to the above two compounds on which we had already done.
On left hand nitrogen atom,
\[LP = 0;BE = 1\]
\[ON = VE - LP - BE\]
\[ON = 5 - 0 - 1 = + 4\]
On right hand nitrogen atom,
\[LP = 0;BE = 1\]
\[ON = VE - LP - BE\]
\[ON = 5 - 0 - 1 = + 4\]
Here both sides are having the same formal charge therefore the oxidation number is definitely \[ + 4\] if we take the average also.
Next we have to take the case of the last compound given which is dinitrogen pentoxide \[({N_2}{O_5})\].
The Lewis structure of \[{N_2}{O_5}\] is

Here also we can know that there are two nitrogen atoms. So , let us follow the same steps which we had already done above in three compounds having two nitrogen atoms.
On left hand side, nitrogen atom it is having as follows,
\[LP = 0;BE = 0\]
\[ON = VE - LP - BE\]
\[ON = 5 - 0 - 0 = + 5\]
On right hand side , nitrogen atom is having as follows,
\[LP = 0;BE = 0\]
\[ON = VE - LP - BE\]
\[ON = 5 - 0 - 0 = + 5\]
Here both sides are having the same formal charge therefore the oxidation number is definitely \[ + 5\] if we take the average also.
In this way Lewis structure is used to to determine the oxidation numbers of \[N\] in compounds like \[{N_2}O\], \[NO\] , \[{N_2}{O_3}\], and \[{N_2}{O_5}\] .
Note: To deal with this type of question first of all we should know about the lewis structure of the compounds because then only we can easily find out the lone pairs and valence electrons in bound atoms which in turn helps to determine the oxidation number. Actually one main thing we have to know is that there is no matter with the bonding which we decide on Lewis dot structures, because in sense we have to destroy that to figure out oxidation states. Also , it makes us always revert back to pretend about ionic structures where electrons are not shared but only transferred to more electronegative atoms. We should follow the rules mentioned in the solution part while determining oxidation number using lewis dot structures so as to find without error.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




