
What is \[2\]- methyl \[1\]- propanol?
Answer
499.5k+ views
Hint: Chemical compounds are classified based on the functional groups. The compounds containing a hydroxyl group can be called an alcohol. The IUPAC name of alcohol can be written as -ol. \[2\]- methyl \[1\]- propanol is an alcohol with the hydroxyl group at \[{1^{st}}\] carbon and methyl substituent at \[{2^{nd}}\] position.
Complete answer: Chemical compounds are huge and classified based on the functional groups present in them. The compounds containing a hydroxyl group are called alcohols. The IUPAC nomenclature of alcohols can be written as -al. The suffix -al can be added to the root word of the carbon atoms.
Given structure has the name of \[2\]- methyl \[1\]- propanol. The name indicates the alcohol at \[{1^{st}}\] carbon and methyl substituent at \[{2^{nd}}\] position.
The structure of \[2\]- methyl \[1\]- propanol is
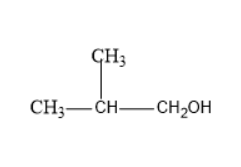
The given compound contains four carbon atoms and one methyl group is in a branched chain. Thus, the root word is propane as the main longest chain consists of three carbon atoms. There is a presence of a methyl group at \[{2^{nd}}\] position. The hydroxyl group is present at \[{1^{st}}\] carbon. Thus, the name is \[2\]- methyl \[1\]- propanol. \[2\]- methyl \[1\]- propanol is an alcohol with a branched methyl group.
Note:
The molecular formula of \[2\]- methyl \[1\]- propanol is \[{C_4}{H_{10}}O\]. Due to the presence of hydroxyl groups adjacent to an alkyl group, it easily undergoes dehydration to form an alkene. The hydroxyl group on one carbon and hydrogen atom on adjacent carbon eliminates as water molecule.
Complete answer: Chemical compounds are huge and classified based on the functional groups present in them. The compounds containing a hydroxyl group are called alcohols. The IUPAC nomenclature of alcohols can be written as -al. The suffix -al can be added to the root word of the carbon atoms.
Given structure has the name of \[2\]- methyl \[1\]- propanol. The name indicates the alcohol at \[{1^{st}}\] carbon and methyl substituent at \[{2^{nd}}\] position.
The structure of \[2\]- methyl \[1\]- propanol is

The given compound contains four carbon atoms and one methyl group is in a branched chain. Thus, the root word is propane as the main longest chain consists of three carbon atoms. There is a presence of a methyl group at \[{2^{nd}}\] position. The hydroxyl group is present at \[{1^{st}}\] carbon. Thus, the name is \[2\]- methyl \[1\]- propanol. \[2\]- methyl \[1\]- propanol is an alcohol with a branched methyl group.
Note:
The molecular formula of \[2\]- methyl \[1\]- propanol is \[{C_4}{H_{10}}O\]. Due to the presence of hydroxyl groups adjacent to an alkyl group, it easily undergoes dehydration to form an alkene. The hydroxyl group on one carbon and hydrogen atom on adjacent carbon eliminates as water molecule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




