
What is a chain reaction? Explain.
Answer
573.6k+ views
Hint: From the name itself we can say that the reactions happen step by step. It is usually done in terms of free radicals. It occurs in mainly five steps like initiation, propagation, and so on. Now, we can explain the chain reaction.
Complete answer:
Let us discuss the chain reaction. As mentioned it occurs step by step, and there is a chain carrier carried further till the reactant is not exhausted completely. In other terms, we can say that there is repetition of steps, and it generates the chain carriers. If we explain the chain reaction in consideration with the mechanism, it consists of five steps
(1)-Initiation step
(2)-Chain propagation step
(3)-Chain branching step
(4)-Chain inhibition step
(5)-Chain termination step
Now, first we will talk about the initiation step; light is used, and it generates free radicals in this step. The second step is the chain propagation step, now when the reaction is initiated, in this step, the number of free radicals used is equal to the generated free radicals.
Now, we have the third step, i.e. chain branching step. It happens when there is formation of more radicals due to the heat, or chain reaction continues, it leads to the chain branching steps. The most important regarding the chain branching step is that it might cause an explosion.
Talking about the fourth step, it is a chain inhibition step. In this step, there is no further formation of products.
The last step we have is the chain termination step. In this step, all the reactants are exhausted, and there is formation of many products.
The example of bromination is given below for better understanding-
Initiation -\[B{{r}_{2}}\xrightarrow{hv}B{{r}^{\bullet }}+B{{r}^{\bullet }}\]
This step mainly forms the radical species. When ultraviolet rays are provided or heat is given, homolytic cleavage takes place. It takes place when both the atoms are of the same polarity.
Propagation-\[B{{r}^{\bullet }}+{{H}_{2}}\to HBr+{{H}^{\bullet }}\]
\[{{H}^{\bullet }}+B{{r}_{2}}\to HBr+B{{r}^{\bullet }}\]
This step basically deals with the propagation step as the name suggests. It leads to generation of more free radicals. It reacts with stable molecules for the formation of new free radicals.
Inhibition-\[{{H}^{\bullet }}+B{{r}_{2}}\to HBr+B{{r}^{\bullet }}\]
Termination-\[B{{r}^{\bullet }}+B{{r}^{\bullet }}+M\to B{{r}_{2}}+{{M}^{*}}\]
This step is basically the ending of a reaction. It occurs when two free radicals combine together and form a stable compound
\[\]
In the last we can conclude that the chain reaction leads to the formation of many products with the help of five steps mentioned.
Note: The problem with free radical reaction is that it is not able to give mono-substituted product as mentioned in the reaction given below-
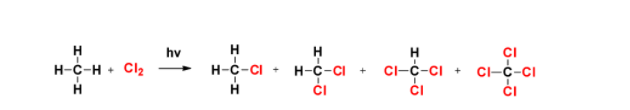
In this reaction, we can see that the formation of mono, di, tri and tetra-chloromethane are formed. One of the ways to reduce the chances of this problem is to use higher concentration of methane with respect to halogens.
Complete answer:
Let us discuss the chain reaction. As mentioned it occurs step by step, and there is a chain carrier carried further till the reactant is not exhausted completely. In other terms, we can say that there is repetition of steps, and it generates the chain carriers. If we explain the chain reaction in consideration with the mechanism, it consists of five steps
(1)-Initiation step
(2)-Chain propagation step
(3)-Chain branching step
(4)-Chain inhibition step
(5)-Chain termination step
Now, first we will talk about the initiation step; light is used, and it generates free radicals in this step. The second step is the chain propagation step, now when the reaction is initiated, in this step, the number of free radicals used is equal to the generated free radicals.
Now, we have the third step, i.e. chain branching step. It happens when there is formation of more radicals due to the heat, or chain reaction continues, it leads to the chain branching steps. The most important regarding the chain branching step is that it might cause an explosion.
Talking about the fourth step, it is a chain inhibition step. In this step, there is no further formation of products.
The last step we have is the chain termination step. In this step, all the reactants are exhausted, and there is formation of many products.
The example of bromination is given below for better understanding-
Initiation -\[B{{r}_{2}}\xrightarrow{hv}B{{r}^{\bullet }}+B{{r}^{\bullet }}\]
This step mainly forms the radical species. When ultraviolet rays are provided or heat is given, homolytic cleavage takes place. It takes place when both the atoms are of the same polarity.
Propagation-\[B{{r}^{\bullet }}+{{H}_{2}}\to HBr+{{H}^{\bullet }}\]
\[{{H}^{\bullet }}+B{{r}_{2}}\to HBr+B{{r}^{\bullet }}\]
This step basically deals with the propagation step as the name suggests. It leads to generation of more free radicals. It reacts with stable molecules for the formation of new free radicals.
Inhibition-\[{{H}^{\bullet }}+B{{r}_{2}}\to HBr+B{{r}^{\bullet }}\]
Termination-\[B{{r}^{\bullet }}+B{{r}^{\bullet }}+M\to B{{r}_{2}}+{{M}^{*}}\]
This step is basically the ending of a reaction. It occurs when two free radicals combine together and form a stable compound
\[\]
In the last we can conclude that the chain reaction leads to the formation of many products with the help of five steps mentioned.
Note: The problem with free radical reaction is that it is not able to give mono-substituted product as mentioned in the reaction given below-

In this reaction, we can see that the formation of mono, di, tri and tetra-chloromethane are formed. One of the ways to reduce the chances of this problem is to use higher concentration of methane with respect to halogens.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




