
What is Popoff’s rule?
Answer
573.6k+ views
Hint: The oxidation of ketones is governed by Popoff’s rule. Unlike aldehydes, ketones do not have any hydrogen atom attached to $>C=O$ group and they cannot be oxidized by weak oxidizing agents such as Tollen’s reagent or Fehling's solution.
Complete Solution :
Popoff’s rule states that during the oxidation of unsymmetrical ketone, the cleavage of the $C-CO$ bond is such that the keto group always stays with the smaller alkyl group.
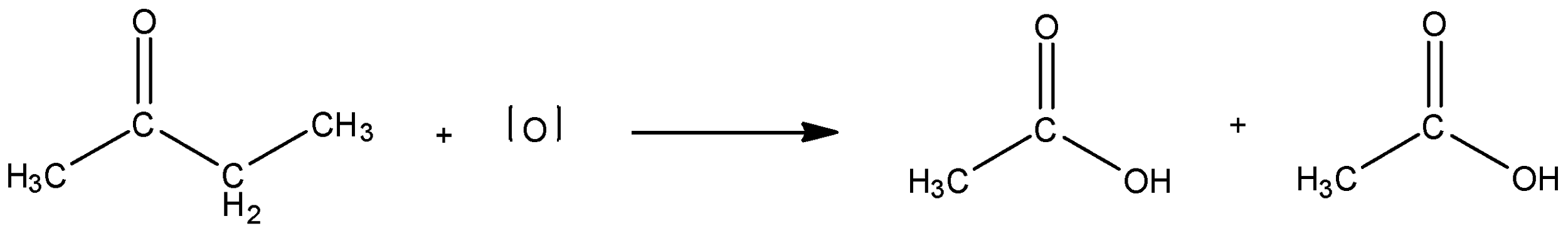
Here is an example, one molecule of ethyl methyl ketone reacts with nascent oxygen to give two molecules of acetic acid.
Here, we see that the initial ethyl methyl ketone has 4 carbons, the products, 2 molecules of acetic acid each have 4 molecules. When the oxidative cleavage of the ketone occurs, the carbonyl carbon stays with the methyl substituent. Both the moieties, the ethyl group and the methyl group along with the keto group, then undergo oxidation to form 2 molecules of acetic acid. Note that the methyl group is the smaller substituent.
Additional Information:
Following are the physical properties of aldehydes and ketones:
- The first member of the aldehyde series, formaldehyde, is a gas while next ten members of the aldehyde series are colourless volatile liquids at ordinary temperature. Ketones up to eleven carbon atoms are also colourless volatile liquids. The higher members of both the series are solids.
- Lower members of aldehyde and ketone series are soluble in water, as they can form hydrogen bonds with water. However, the solubility decreases as the molecular mass increases. The members having more than five carbon atoms are somewhat insoluble. These compounds are freely soluble in organic solvents such as alcohol, ether, etc.
Note: Because ketones do not have any particular hydrogen atom at the carbonyl carbon, they are resistant to oxidation. Ketones do undergo oxidation reactions at extreme temperatures.
Complete Solution :
Popoff’s rule states that during the oxidation of unsymmetrical ketone, the cleavage of the $C-CO$ bond is such that the keto group always stays with the smaller alkyl group.
Here is an example, one molecule of ethyl methyl ketone reacts with nascent oxygen to give two molecules of acetic acid.
Here, we see that the initial ethyl methyl ketone has 4 carbons, the products, 2 molecules of acetic acid each have 4 molecules. When the oxidative cleavage of the ketone occurs, the carbonyl carbon stays with the methyl substituent. Both the moieties, the ethyl group and the methyl group along with the keto group, then undergo oxidation to form 2 molecules of acetic acid. Note that the methyl group is the smaller substituent.

Additional Information:
Following are the physical properties of aldehydes and ketones:
- The first member of the aldehyde series, formaldehyde, is a gas while next ten members of the aldehyde series are colourless volatile liquids at ordinary temperature. Ketones up to eleven carbon atoms are also colourless volatile liquids. The higher members of both the series are solids.
- Lower members of aldehyde and ketone series are soluble in water, as they can form hydrogen bonds with water. However, the solubility decreases as the molecular mass increases. The members having more than five carbon atoms are somewhat insoluble. These compounds are freely soluble in organic solvents such as alcohol, ether, etc.
Note: Because ketones do not have any particular hydrogen atom at the carbonyl carbon, they are resistant to oxidation. Ketones do undergo oxidation reactions at extreme temperatures.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




