
What is the bond angle of $Si{O_2}{\text{ ?}}$
Answer
503.4k+ views
Hint: Bond angle may be defined as the angle formed between the two covalent bonds originating from the same atom. According to valence shell electron pair repulsion theory we can predict the geometry of the molecule. Bond angle can also be predicted using the geometry of the molecule.
Complete answer:
Silicon Dioxide is formed by combining one silicon atom and two oxygen atoms. Silicon has atomic number $1$. Therefore its electronic configuration can be written as $2,8,4$. Thus it has four valence electrons. Therefore it will form four bonds with oxygen. Therefore it can be depict as:
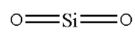
Here there are only two sigma bonds present. Hence it will be $sp$ hybridized. Therefore we know that the bond angle for $sp$ hybridized molecule is ${180^ \circ }$.
But in actual silicon dioxide is not a single molecule. Silicon Dioxide exists in a lattice structure where silicon forms four sigma bonds with four oxygen atoms. This lattice structure is the polymer structure of the silicon dioxide. Hence the lattice structure can be depicted as:
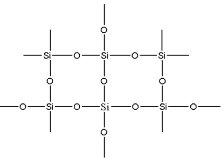
Therefore every silicon atom is connected to four oxygen atoms by a sigma bond. Thus here the hybridization of each silicon atom is ${109.5^ \circ }$.
Note:
The given lattice structure of silicon dioxide is a snap of the whole structure. The given structure is polymerized to produce the whole structure. The valency of each atom silicon and oxygen is satisfied in the lattice structure. This is the stable structure of silicon dioxide. The molecule which has ${109.5^ \circ }$ has tetrahedral geometry according to VSEPR. Also there is a lone pair of an electron present on the oxygen atom which may also distort its geometry.
Complete answer:
Silicon Dioxide is formed by combining one silicon atom and two oxygen atoms. Silicon has atomic number $1$. Therefore its electronic configuration can be written as $2,8,4$. Thus it has four valence electrons. Therefore it will form four bonds with oxygen. Therefore it can be depict as:
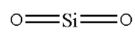
Here there are only two sigma bonds present. Hence it will be $sp$ hybridized. Therefore we know that the bond angle for $sp$ hybridized molecule is ${180^ \circ }$.
But in actual silicon dioxide is not a single molecule. Silicon Dioxide exists in a lattice structure where silicon forms four sigma bonds with four oxygen atoms. This lattice structure is the polymer structure of the silicon dioxide. Hence the lattice structure can be depicted as:

Therefore every silicon atom is connected to four oxygen atoms by a sigma bond. Thus here the hybridization of each silicon atom is ${109.5^ \circ }$.
Note:
The given lattice structure of silicon dioxide is a snap of the whole structure. The given structure is polymerized to produce the whole structure. The valency of each atom silicon and oxygen is satisfied in the lattice structure. This is the stable structure of silicon dioxide. The molecule which has ${109.5^ \circ }$ has tetrahedral geometry according to VSEPR. Also there is a lone pair of an electron present on the oxygen atom which may also distort its geometry.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




