
What is the enol of ethanal?
Answer
493.2k+ views
Hint: Enol is the organic compound that comprises both an alkene (-en) and an alcohol functional group (-OH). Hence it is known as enol. Enols are usually formed as a product of tautomerism. This tautomerism is known as keto enol tautomerism.
Complete Step By Step Answer:
An aldehyde with at least one hydrogen on the carbon atom next to the carbonyl group i.e. $ \alpha $ Hydrogen atom, this hydrogen can migrate to the carbonyl oxygen. The double bond formed migrates to the $ \alpha $ carbon. Therefore, an aldehyde with at least one $ \alpha $ hydrogen can tautomerize and can exist in two isomeric forms. These are known as Tautomers. In aldehyde form the hydrogen is bonded to the $ \alpha $ carbon and in enol form it is bonded to the oxygen attached to the carbonyl group, with the migration of the double bond.
Keto and enol isomers exist in equilibrium, but normally the keto/also form is more stable than the enol form. For the given molecule of acetaldehyde 6 in 10 million molecules exists in enol form. The equilibrium exists between the two, and all the ketones and aldehyde with a $ \alpha $ hydrogen exist in enol form. Every enol form is converted to the keto/aldo form several times in a second. The tautomerism of ethanal or acetaldehyde to its enol can be given as follows:
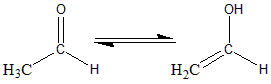
The IUPAC name of the enol form is Eth-1-ene-1-ol.
Note:
The formation of enols from keto forms is an important characteristic because a number of reactions takes place via the enol form only. The percentage of enol form is higher than the keto form in some carbonyl compounds.
Complete Step By Step Answer:
An aldehyde with at least one hydrogen on the carbon atom next to the carbonyl group i.e. $ \alpha $ Hydrogen atom, this hydrogen can migrate to the carbonyl oxygen. The double bond formed migrates to the $ \alpha $ carbon. Therefore, an aldehyde with at least one $ \alpha $ hydrogen can tautomerize and can exist in two isomeric forms. These are known as Tautomers. In aldehyde form the hydrogen is bonded to the $ \alpha $ carbon and in enol form it is bonded to the oxygen attached to the carbonyl group, with the migration of the double bond.
Keto and enol isomers exist in equilibrium, but normally the keto/also form is more stable than the enol form. For the given molecule of acetaldehyde 6 in 10 million molecules exists in enol form. The equilibrium exists between the two, and all the ketones and aldehyde with a $ \alpha $ hydrogen exist in enol form. Every enol form is converted to the keto/aldo form several times in a second. The tautomerism of ethanal or acetaldehyde to its enol can be given as follows:

The IUPAC name of the enol form is Eth-1-ene-1-ol.
Note:
The formation of enols from keto forms is an important characteristic because a number of reactions takes place via the enol form only. The percentage of enol form is higher than the keto form in some carbonyl compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




