
Which among the following is the correct IUPAC name of isoamylene?
(a) ${\text{Pent - 1 - ene}} $
(b) $2 - {\text{Methylbut - 2 - ene}} $
(c) $3 - {\text{Methylbut - 1 - ene}} $
(d) $2 - {\text{Methylbut - 1 - ene}} $
Answer
512.7k+ views
Hint :To know organic compounds, we have to memorize a few basic points. The base part of the names reflects the number of carbon atoms present in the parent chain. The suffix of the name reflects the type of functional group that is present in the parent chain.
Complete Step By Step Answer:
If a hydrocarbon has a double bond in it, the suffix $ - {\text{ene}} $ is used. The position of the double bond in the parent chain is indicated by placing the number of the first carbon of the double bond directly in front of the base name. The structure of isoamylene is as follows:
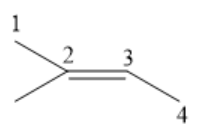
Here, the longest possible parent chain will have four carbon atoms. Give numbering to the carbon atoms in the parent chain. There is a double bond in the hydrocarbon, so the suffix $ - {\text{ene}} $ should be used. The double bond is at position $2 $. We should write the position of the double bond in front of the base name. As the parent chain has four carbon atoms, so we use $ - {\text{but}} $. There is a methyl group attached to the second carbon atom, so the IUPAC name will be: $2 - {\text{Methylbut - 2 - ene}} $
Therefore, option B is the correct answer.
Note :
Remember to number the parent chain in such a way that the multiple bonds have the lowest numbers. If there is more than one double bond in the given hydrocarbon, the suffix is generally expanded to include a prefix indicating the number of double bonds present in the hydrocarbon.
Complete Step By Step Answer:
If a hydrocarbon has a double bond in it, the suffix $ - {\text{ene}} $ is used. The position of the double bond in the parent chain is indicated by placing the number of the first carbon of the double bond directly in front of the base name. The structure of isoamylene is as follows:

Here, the longest possible parent chain will have four carbon atoms. Give numbering to the carbon atoms in the parent chain. There is a double bond in the hydrocarbon, so the suffix $ - {\text{ene}} $ should be used. The double bond is at position $2 $. We should write the position of the double bond in front of the base name. As the parent chain has four carbon atoms, so we use $ - {\text{but}} $. There is a methyl group attached to the second carbon atom, so the IUPAC name will be: $2 - {\text{Methylbut - 2 - ene}} $
Therefore, option B is the correct answer.
Note :
Remember to number the parent chain in such a way that the multiple bonds have the lowest numbers. If there is more than one double bond in the given hydrocarbon, the suffix is generally expanded to include a prefix indicating the number of double bonds present in the hydrocarbon.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




