
Which among the following molecules can exhibit tautomerism?
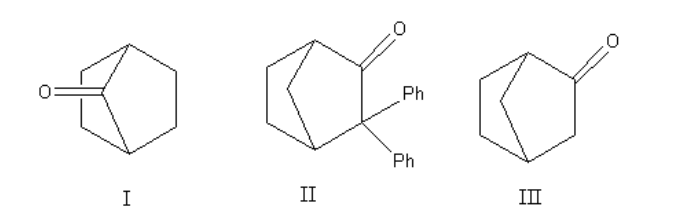
A. Both I and II
B. Both II and III
C. III only
D. Both I and III

Answer
571.8k+ views
Hint:Tautomers are structural isomers of each other. They differed from each other in the position of the proton and electron. Ketone functional group having \[\alpha \] hydrogen undergoes keto-enol tautomerism. All the given molecules are bridge compounds so use Bredt’s rule and determine which molecule undergoes tautomerism.
Complete answer:
Keto-enol tautomerism is the conversion of a ketone into enol form. There is a chemical equilibrium between both forms.
First, we will write the possible enol isomers for all three molecules.
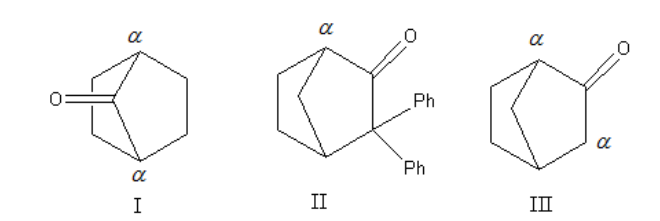
To convert keto form to enol form compound should contain \[\alpha \] hydrogen. So we will assign the \[\alpha \] hydrogen to all three structures.
Now, we will write the tautomerism reaction for all three compounds and will predict the possible products.
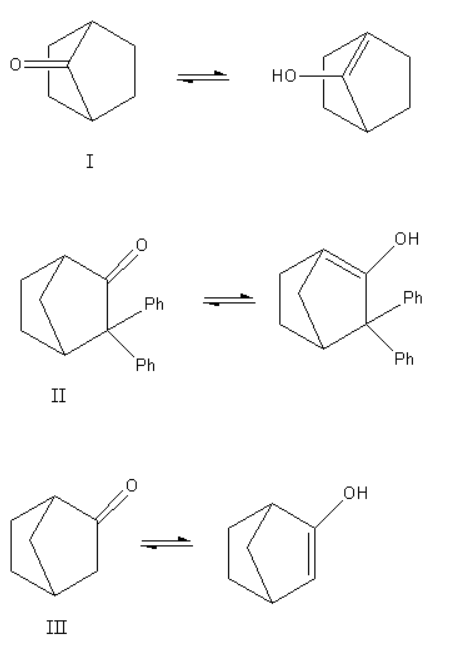
Here, we can see that structure I and II give the bridgehead double bond. According to Bredt’s rule in the case of a bridged ring, the bridgehead double bond does not exist as the bridgehead double bond is unstable. As the enols of structure, I and II have bridgehead double bonds they do not exist. Only structure III gives a non-bridgehead double bond so it shows keto-enol tautomerism.
Thus, out of the three structures only structure III exhibit tautomerism.
So, the correct option is (C) III only.
Note:
The molecule should obey two conditions to undergo tautomerism. The first condition is that the molecule should have \[\alpha \] hydrogen. The second condition is for bridge ring molecules. If the molecule is a bridged ring the double bond should not be bridgehead.
Complete answer:
Keto-enol tautomerism is the conversion of a ketone into enol form. There is a chemical equilibrium between both forms.
First, we will write the possible enol isomers for all three molecules.
To convert keto form to enol form compound should contain \[\alpha \] hydrogen. So we will assign the \[\alpha \] hydrogen to all three structures.

Now, we will write the tautomerism reaction for all three compounds and will predict the possible products.

Here, we can see that structure I and II give the bridgehead double bond. According to Bredt’s rule in the case of a bridged ring, the bridgehead double bond does not exist as the bridgehead double bond is unstable. As the enols of structure, I and II have bridgehead double bonds they do not exist. Only structure III gives a non-bridgehead double bond so it shows keto-enol tautomerism.
Thus, out of the three structures only structure III exhibit tautomerism.
So, the correct option is (C) III only.
Note:
The molecule should obey two conditions to undergo tautomerism. The first condition is that the molecule should have \[\alpha \] hydrogen. The second condition is for bridge ring molecules. If the molecule is a bridged ring the double bond should not be bridgehead.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




