
Which compound has planar structure?
(A) $Xe{F_4}$
(B) $XeO{F_2}$
(C) $Xe{O_2}{F_2}$
(D) $Xe{O_4}$
Answer
587.7k+ views
Hint: Any compound is said to be planar only when all atoms of a molecule lie in the same plane. Here, we first calculate the hybridization of the given compound and then we will predict the shape based on the hybridization obtained.
Complete step by step answer:
$Xe{F_4}$ is known to have square planar structure.
At first we will calculate the hybridisation of $Xe{F_4}$
The central atom is \[Xe\]. In the valence shell of \[Xe\], there are six electrons in \[5p\] orbital and \[2\] electrons in the \[5s\] orbital. There are \[5d\] and \[5f\] orbitals left that are empty.
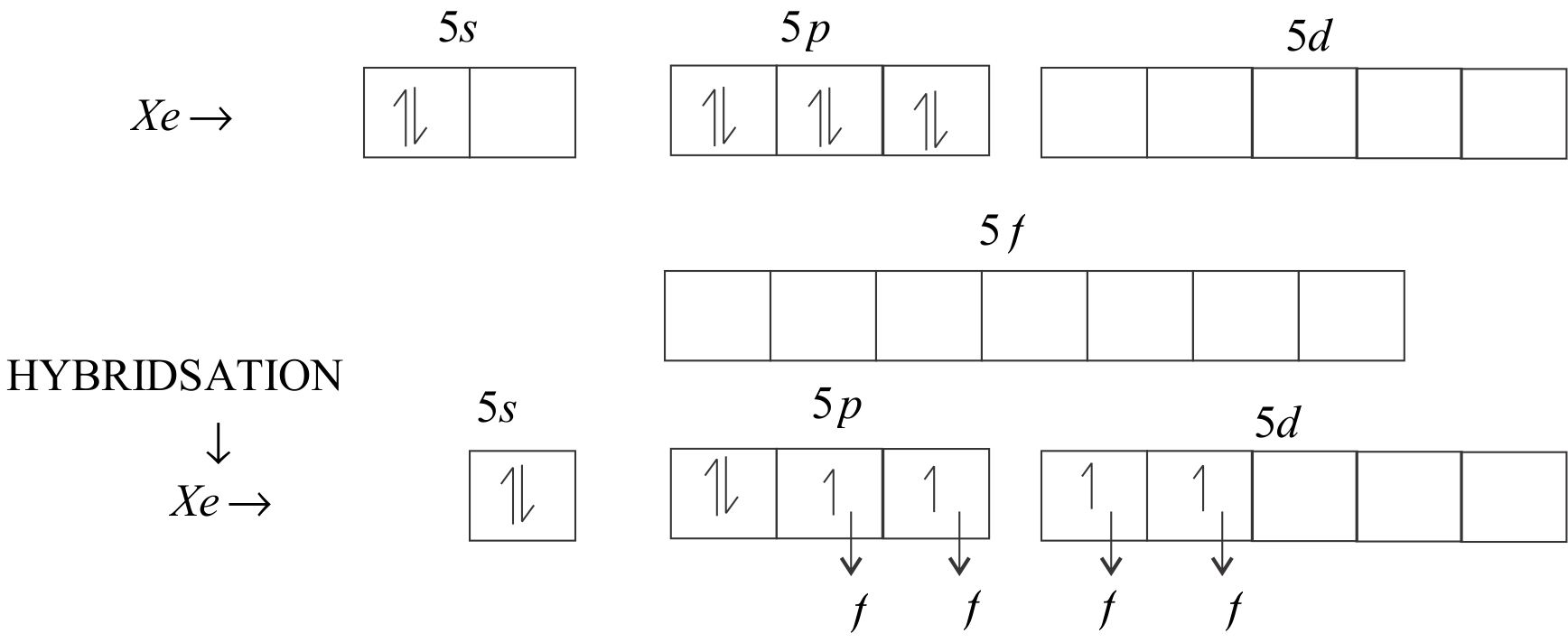
\[s{p^3}{d^2}\] will be the hybridization of $Xe{F_4}$.
So, the hybridisation is \[s{p^3}{d^2}\] which contains two lone pairs and four bond pairs orbitals.
So, the geometry of $Xe{F_4}$
So, the molecule is square planar.
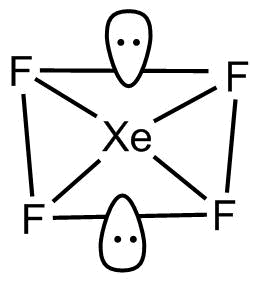
The two lone pairs are present upside down and the other four are bonded with fluorine atoms.
Additional information:
Xenon tetrafluoride is produced as a result for the reaction of xenon with fluorine. The reaction equation can be written as:
${\text{Xe + 2}}{{\text{F}}_{\text{2}}} \to {\text{Xe}}{{\text{F}}_{\text{4}}}$
Xenon tetrafluoride is a colorless crystalline substance. Looking at the structure of xenon tetrafluoride, it has two lone pairs of electrons in addition with the four fluorine ligands in accordance with the VSEPR theory. The lone pairs are actually mutually trans.
Xenon tetrafluoride found to have certain few applications. It is used in reaction with silicon to form simple gaseous products that leaves behind a residue of metal impurities. It has also been used to degrade silicone rubber to analyze the impurities in the rubber.
Note:
It should be noted that the central atom that is xenon has six electron groups out of which the two of them are lone pairs. the hybridization of any atom must be predicted carefully keeping in mind the no. of lone pairs present.
Complete step by step answer:
$Xe{F_4}$ is known to have square planar structure.
At first we will calculate the hybridisation of $Xe{F_4}$
The central atom is \[Xe\]. In the valence shell of \[Xe\], there are six electrons in \[5p\] orbital and \[2\] electrons in the \[5s\] orbital. There are \[5d\] and \[5f\] orbitals left that are empty.

\[s{p^3}{d^2}\] will be the hybridization of $Xe{F_4}$.
So, the hybridisation is \[s{p^3}{d^2}\] which contains two lone pairs and four bond pairs orbitals.
So, the geometry of $Xe{F_4}$
So, the molecule is square planar.

The two lone pairs are present upside down and the other four are bonded with fluorine atoms.
Additional information:
Xenon tetrafluoride is produced as a result for the reaction of xenon with fluorine. The reaction equation can be written as:
${\text{Xe + 2}}{{\text{F}}_{\text{2}}} \to {\text{Xe}}{{\text{F}}_{\text{4}}}$
Xenon tetrafluoride is a colorless crystalline substance. Looking at the structure of xenon tetrafluoride, it has two lone pairs of electrons in addition with the four fluorine ligands in accordance with the VSEPR theory. The lone pairs are actually mutually trans.
Xenon tetrafluoride found to have certain few applications. It is used in reaction with silicon to form simple gaseous products that leaves behind a residue of metal impurities. It has also been used to degrade silicone rubber to analyze the impurities in the rubber.
Note:
It should be noted that the central atom that is xenon has six electron groups out of which the two of them are lone pairs. the hybridization of any atom must be predicted carefully keeping in mind the no. of lone pairs present.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




