
Which compounds were used by Miller in his experiment for obtaining amino acid and other organic substances
(a)Carbon dioxide, water vapour and methane
(b)Methane, ammonia, water vapour and hydrogen cyanide
(c)Ammonia, methane, hydrogen and water vapour
(d)Ammonia, methane and carbon dioxide
Answer
567.3k+ views
Hint: The condition inside the system of Miller's experiment was aided through certain compounds which resembled the atmosphere present on the early earth. Electric current was also passed through this system, to simulate lightning storms that were also believed to be common on the early earth.
Complete answer:
The Miller experiment that imitated the conditions thought at the time (1952) to be present on the early Earth was mainly a chemical experiment. Certain chemicals that were known to support 'origin of life' under those conditions on earth were tested. Alexander Oparin and J. B. S. Haldane supported the hypothesis at that time. According to them ancient conditions on the primitive Earth favoured certain chemical reactions that enabled synthesis of more complex organic compounds from simpler inorganic precursors. The main experiment was performed in 1952 by Stanley Miller and Harold Urey as the supervisor at the University of Chicago.
Additional Information: The experiment used compounds such as water, methane, ammonia, and hydrogen. The above-mentioned chemicals were all sealed inside a sterile glass flask connected to another flask half-full of water. The water in the smaller flask was heated that induced evaporation and the water vapour was allowed to enter the larger flask. Continuous electrical sparks were fired through tungsten electrodes to simulate lightning in the water vapour and gaseous mixture. In the next step, the temperature in the simulated atmosphere was lowered again so that the condensed water can trickle into a U-shaped trap at the bottom of the apparatus.
After one day, the collected solution had turned pink in colour, and after a week of continuous operation, the solution was deep red and turbid. The reaction was closed by adding barium hydroxide and sulfuric acid. The mixture was then evaporated to remove impurities. Using the method of paper chromatography, Miller identified five amino acids that were present in the solution: glycine, α-alanine and β-alanine, aspartic acid and α-aminobutyric acid (AABA).
So, the correct answer is option c.
Note: Due to abundant evidence of major volcanic eruptions 4 billion years ago, it is estimated that carbon dioxide, nitrogen, hydrogen sulfide, and sulfur dioxide were released into the atmosphere. The experiments that were carried out later using these gases along with the gases used in Miller's experiment produced more diverse molecules.
Complete answer:
The Miller experiment that imitated the conditions thought at the time (1952) to be present on the early Earth was mainly a chemical experiment. Certain chemicals that were known to support 'origin of life' under those conditions on earth were tested. Alexander Oparin and J. B. S. Haldane supported the hypothesis at that time. According to them ancient conditions on the primitive Earth favoured certain chemical reactions that enabled synthesis of more complex organic compounds from simpler inorganic precursors. The main experiment was performed in 1952 by Stanley Miller and Harold Urey as the supervisor at the University of Chicago.
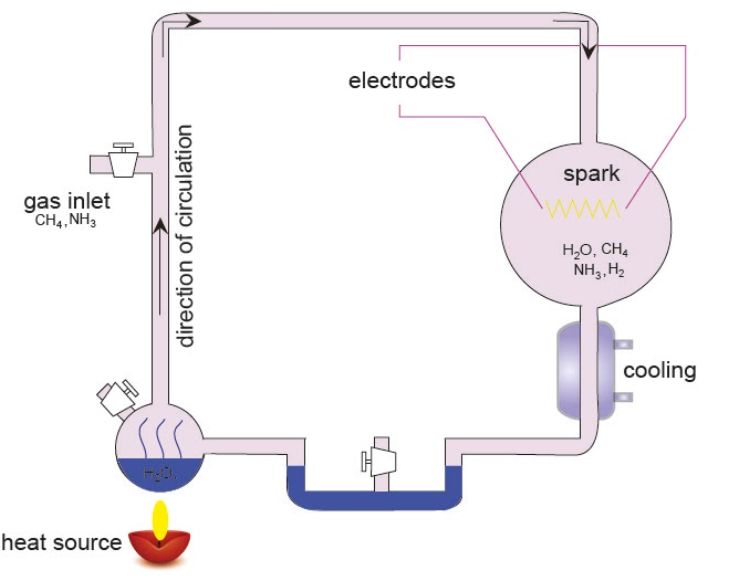
Additional Information: The experiment used compounds such as water, methane, ammonia, and hydrogen. The above-mentioned chemicals were all sealed inside a sterile glass flask connected to another flask half-full of water. The water in the smaller flask was heated that induced evaporation and the water vapour was allowed to enter the larger flask. Continuous electrical sparks were fired through tungsten electrodes to simulate lightning in the water vapour and gaseous mixture. In the next step, the temperature in the simulated atmosphere was lowered again so that the condensed water can trickle into a U-shaped trap at the bottom of the apparatus.
After one day, the collected solution had turned pink in colour, and after a week of continuous operation, the solution was deep red and turbid. The reaction was closed by adding barium hydroxide and sulfuric acid. The mixture was then evaporated to remove impurities. Using the method of paper chromatography, Miller identified five amino acids that were present in the solution: glycine, α-alanine and β-alanine, aspartic acid and α-aminobutyric acid (AABA).
So, the correct answer is option c.
Note: Due to abundant evidence of major volcanic eruptions 4 billion years ago, it is estimated that carbon dioxide, nitrogen, hydrogen sulfide, and sulfur dioxide were released into the atmosphere. The experiments that were carried out later using these gases along with the gases used in Miller's experiment produced more diverse molecules.

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




