
Which is a pair of geometrical isomers?
(1)
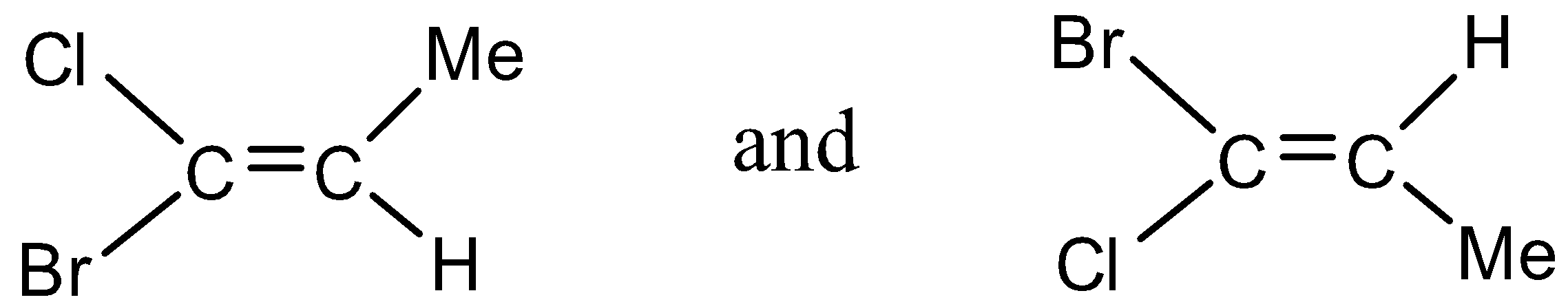
(2)
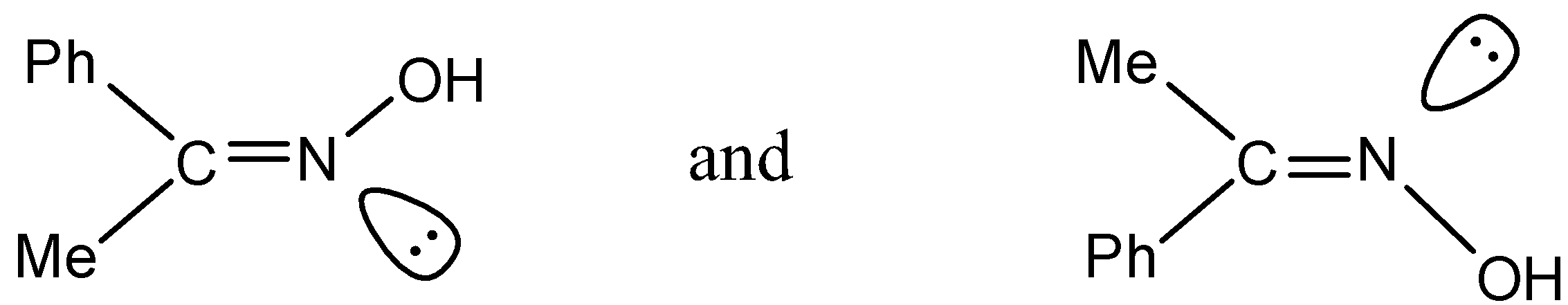
(3)
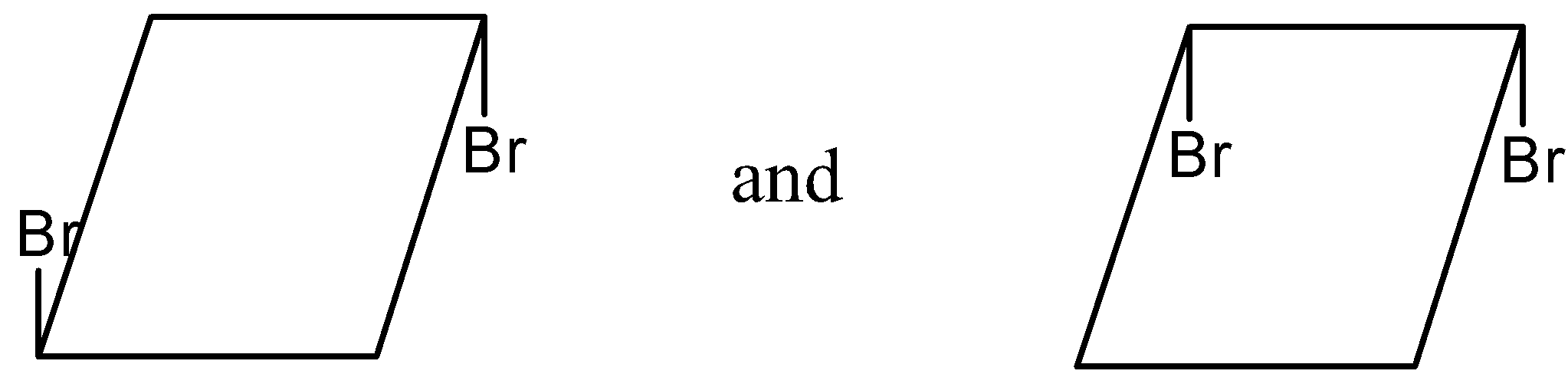
(4)
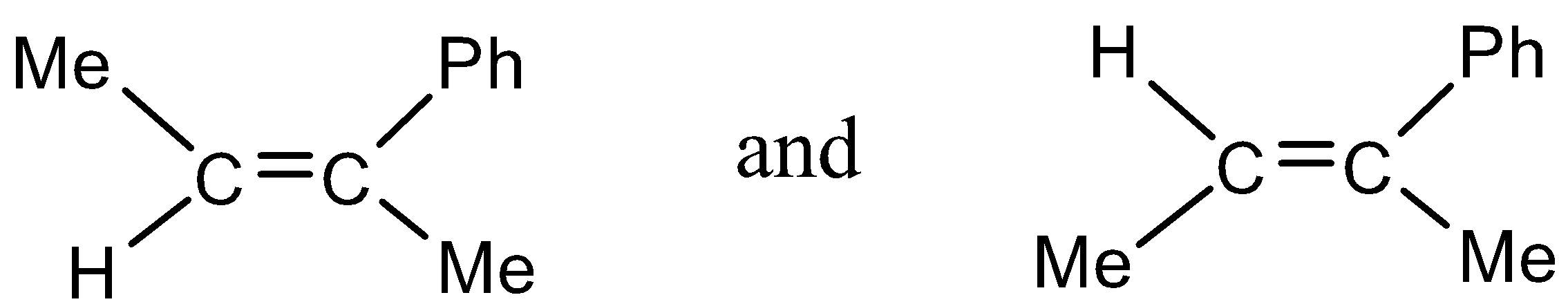
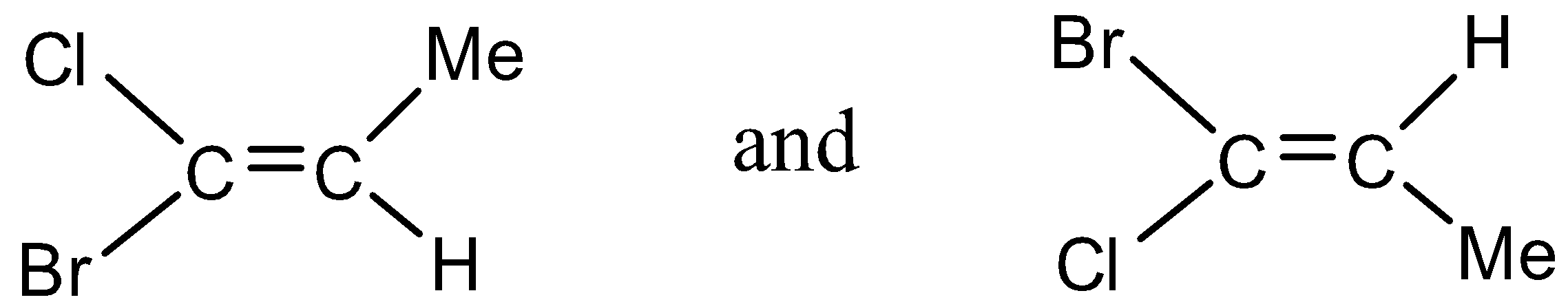
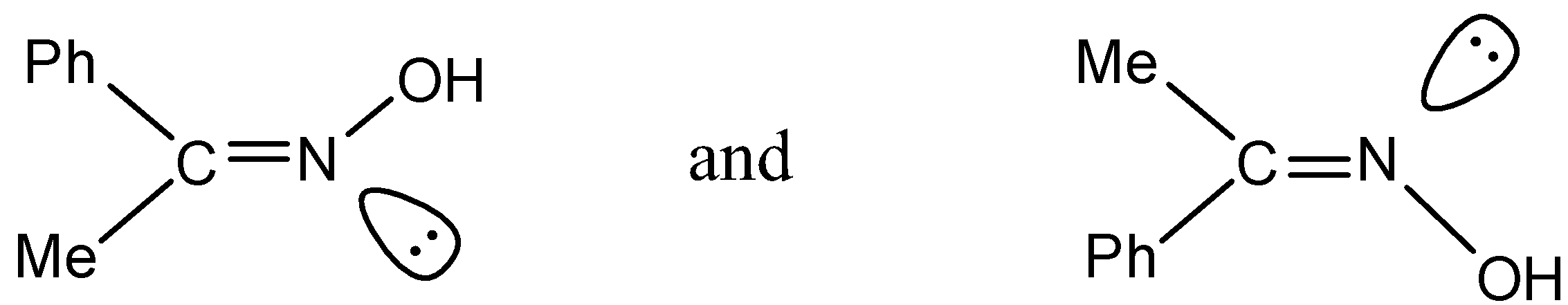
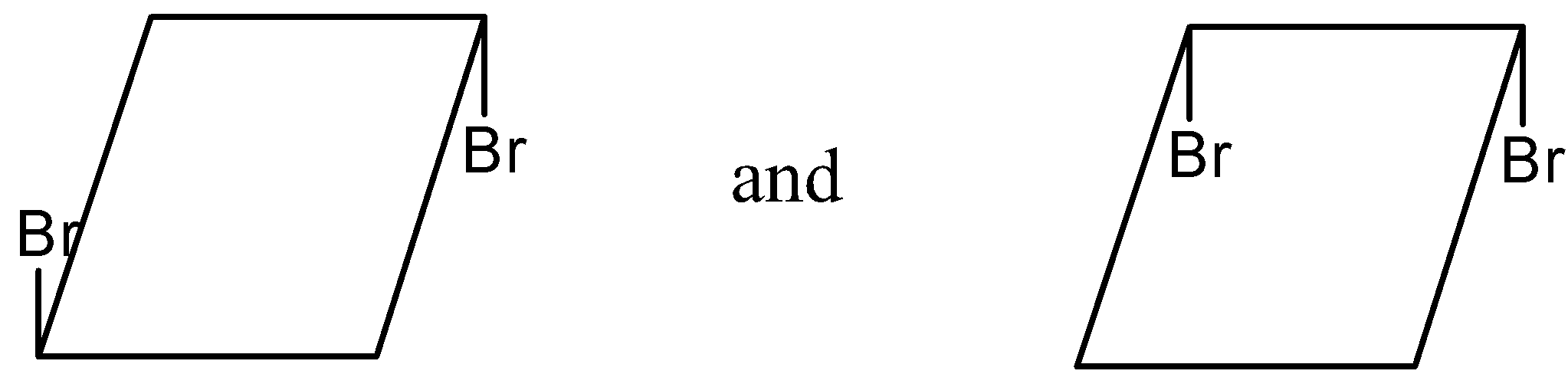
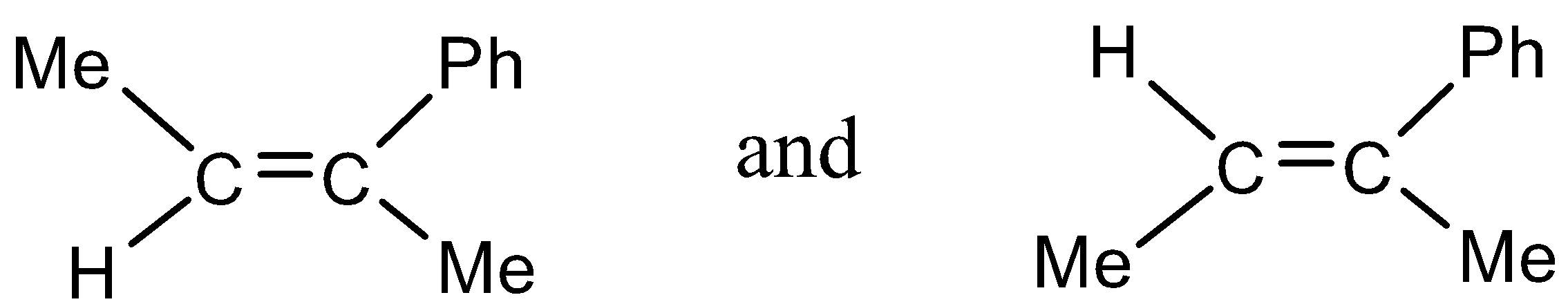
Answer
561.3k+ views
Hint The answer lies in the concept of organic chemistry which deals with the stereochemistry part where the geometrical isomers are the cis – trans pairs. This will give you the required answer.
Complete step – by – step answer:
The concept of stereochemistry which deals with the study of structures of a molecule in space is familiar to us which comes under the organic chemistry part.
Let us now see in detail about the geometrical isomerism and how it can be identified when a pair of molecules is given so that we will be able to deduce the correct answer.
- Stereochemistry is the study of the molecules in space which tells about the arrangement of atoms in a molecule in space. Thus, this is also called as 3D chemistry as the molecules are studied in the 3 dimensional form.
- Isomers are the molecules with the same chemical formula but different spatial arrangement of atoms in a molecule in space.
- There are different types of isomerism where in geometrical isomerism is nothing but the pair of cis – trans isomers.
- The main requirement for this is that in a given molecule there should at least be two same atoms each of which are attached to two different carbons in a single molecule.
- In option (1)
The molecule contains all the atoms which are different and hence the cis – trans pair does not occur. This option is ruled out.
- In option (2)
Even here there are no two same atoms and therefore this option is ruled out.
- In option (3)
The two same atoms that is bromine is present at different carbon atoms in the given two molecule and thus the cis or trans pair is not seen here and this option is ruled out.
- In option (4)
There are two methyl groups which are the same atoms in a single molecule and also in the first structure the methyl groups are trans to each other and in the second structure the two methyl groups are cis to each other.
Thus, option (4) is the correct answer.
Note: Note that only alkenes can have cis or trans isomers because of their rigid bond and there is restricted rotation of the double bond because of the pi bond which means that they don’t inter covert readily and hence they exist as stereoisomers
Complete step – by – step answer:
The concept of stereochemistry which deals with the study of structures of a molecule in space is familiar to us which comes under the organic chemistry part.
Let us now see in detail about the geometrical isomerism and how it can be identified when a pair of molecules is given so that we will be able to deduce the correct answer.
- Stereochemistry is the study of the molecules in space which tells about the arrangement of atoms in a molecule in space. Thus, this is also called as 3D chemistry as the molecules are studied in the 3 dimensional form.
- Isomers are the molecules with the same chemical formula but different spatial arrangement of atoms in a molecule in space.
- There are different types of isomerism where in geometrical isomerism is nothing but the pair of cis – trans isomers.
- The main requirement for this is that in a given molecule there should at least be two same atoms each of which are attached to two different carbons in a single molecule.
- In option (1)
The molecule contains all the atoms which are different and hence the cis – trans pair does not occur. This option is ruled out.
- In option (2)
Even here there are no two same atoms and therefore this option is ruled out.
- In option (3)
The two same atoms that is bromine is present at different carbon atoms in the given two molecule and thus the cis or trans pair is not seen here and this option is ruled out.
- In option (4)
There are two methyl groups which are the same atoms in a single molecule and also in the first structure the methyl groups are trans to each other and in the second structure the two methyl groups are cis to each other.
Thus, option (4) is the correct answer.
Note: Note that only alkenes can have cis or trans isomers because of their rigid bond and there is restricted rotation of the double bond because of the pi bond which means that they don’t inter covert readily and hence they exist as stereoisomers
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




