
Which is the correct structural formula for terylene?
a)
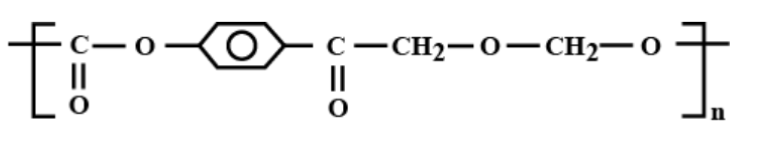
b)
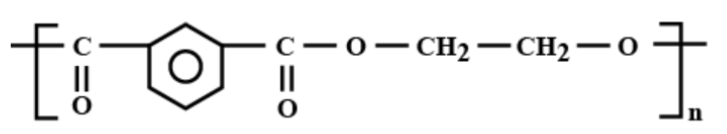
c)
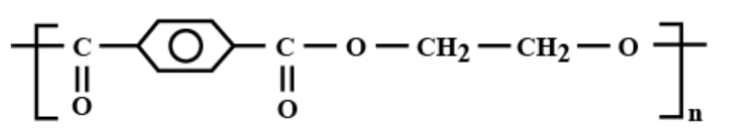
d)
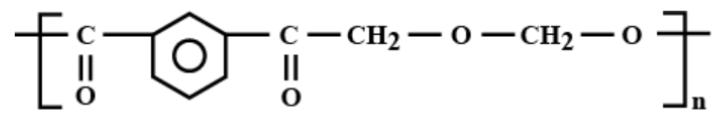
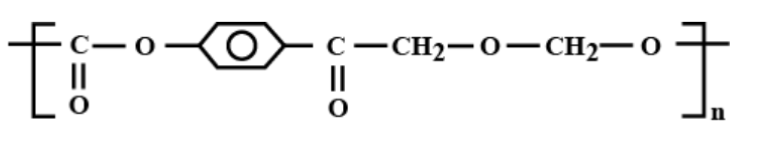
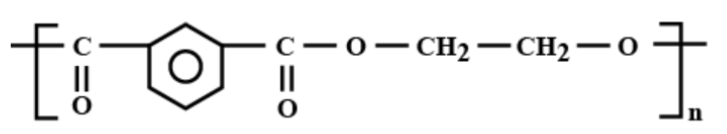
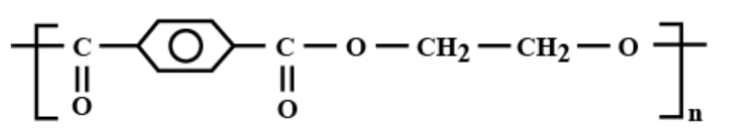
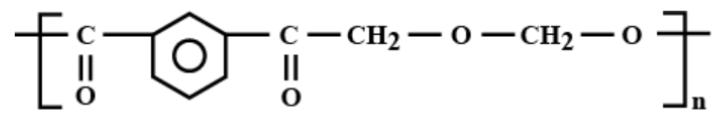
Answer
600k+ views
Hint: It is extensively used in the textile industry to make clothes like sarees, tapestry and dress material. It can also be mixed with natural fibers like cotton and wool to make more variety of clothes.
Complete step by step solution:
Before we start answering we need to know what condensation polymerization means and what is a polyester.
During the process of polymeric chain growth if chemical reaction takes place between different functional groups of single monomer or different monomers with the loss of compounds other than used monomer units then the polymerization process is called condensation polymerization. Examples of condensation polymers are PF, UF, MF, Nylon, PET etc.
Polyester is a polymer having a poly ester group(-COOC-) in a long back bone chain. A polyester is made by a reaction involving an acid with two -COOH groups, and an alcohol with two -OH groups.
Terylene is a synthetic polyester made in 1941 by chemist J R WHINFIELD. It was prepared by the process of condensation polymerization of equimolar ratio ethylene glycol (ethane-1,2-diol) and terephthalic acid (benzene-1,4-dicarboxylic acid) at 140 − 190℃ in the presence of zinc acetate-antimony trioxide catalyst.
The acid is terephthalic acid (benzene-1,4-dicarboxylic).
The alcohol is ethylene glycol (ethane-1,2-diol).
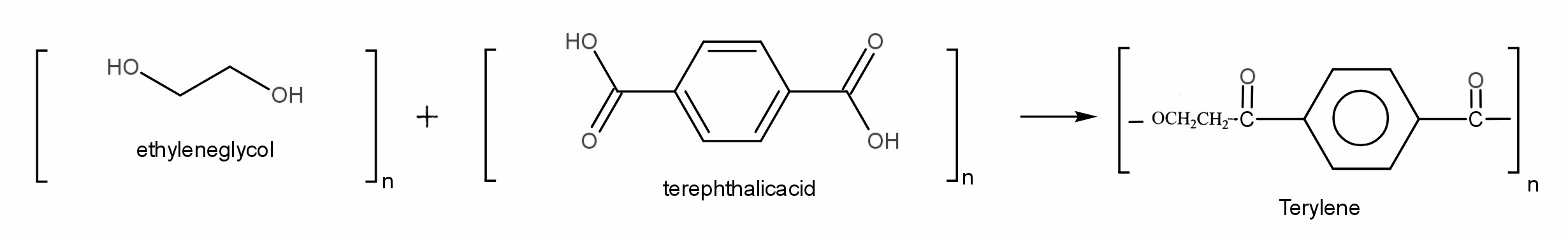
Thus, the correct answer is (c).
Note: There is an intermediate step in between called transesterification after which the polymerization step is done. Water is formed as a by-product of the condensation polymerization of the monomers.
Complete step by step solution:
Before we start answering we need to know what condensation polymerization means and what is a polyester.
During the process of polymeric chain growth if chemical reaction takes place between different functional groups of single monomer or different monomers with the loss of compounds other than used monomer units then the polymerization process is called condensation polymerization. Examples of condensation polymers are PF, UF, MF, Nylon, PET etc.
Polyester is a polymer having a poly ester group(-COOC-) in a long back bone chain. A polyester is made by a reaction involving an acid with two -COOH groups, and an alcohol with two -OH groups.
Terylene is a synthetic polyester made in 1941 by chemist J R WHINFIELD. It was prepared by the process of condensation polymerization of equimolar ratio ethylene glycol (ethane-1,2-diol) and terephthalic acid (benzene-1,4-dicarboxylic acid) at 140 − 190℃ in the presence of zinc acetate-antimony trioxide catalyst.
The acid is terephthalic acid (benzene-1,4-dicarboxylic).
The alcohol is ethylene glycol (ethane-1,2-diol).

Thus, the correct answer is (c).
Note: There is an intermediate step in between called transesterification after which the polymerization step is done. Water is formed as a by-product of the condensation polymerization of the monomers.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Who is eligible for RTE class 9 social science CBSE

What is the Full Form of ISI and RAW

How do you find the valency of chlorine sulphur and class 9 chemistry CBSE

What are the major achievements of the UNO class 9 social science CBSE

Explain the importance of pH in everyday life class 9 chemistry CBSE

Differentiate between parenchyma collenchyma and sclerenchyma class 9 biology CBSE




