
Which of the following amino acids has a hydroxyl methyl group as its R group?
A. Serine
B. Proline
C. Alanine
D. Arginine
Answer
579.9k+ views
Hint: Amino acids are the monomeric units of proteins. Proteins like DNA, RNA is made of amino acids. There are different kind of amino acids, which are, \[\alpha - amino\,acids,\beta - amino\,acids,\gamma - amino\,acids\,etc\] .The name of the amino acids is based on positions of the group attached to the carbon atoms of the molecules
Complete step by step answer:
Among all kinds of amino acids \[\alpha \] - amino acids are very much important. Among all amino acids there are some essential amino acids and non-essential amino acids.
Those amino acids which cannot be prepared inside the body are known as essential amino acids. For example, leucine, lysine, histidine, etc. And those amino acids which can be produced in our body are called non-essential amino acids. For example, aspartic acid, glycine, proline, etc.
If any amino acids have an extra amine group then it is called basic amino acid and if any amino acid has an extra acidic group then it is called an acidic amino acid. The side chain (R group) is unique for every amino acid. Depending upon the side-chain R group amino acid can be hydrophilic or hydrophobic.
Among the given options serine has a hydroxyl methyl group in this side chain. The structure of serine is as follows,
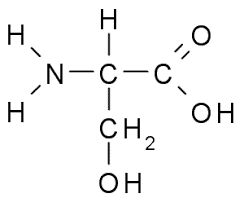
So, the correct option is A.
Note: The isoelectric point of the amino acid is the pH at which a molecule carries no net electric charge or is electrically neutral in the statistical mean. Consider option A and B, at \[pH = 5.97\] glycine does not migrate while lysine moves to the cathode. At this pH, glycine is present in the form of zwitterion, as the pH is equal to the isoelectric point of glycine. At \[pH = 9.6\] , lysine does not migrate while glycine moves to the anode. At this pH, lysine is present in the form of zwitterion as the pH is equal to the isoelectric point of lysine.
Complete step by step answer:
Among all kinds of amino acids \[\alpha \] - amino acids are very much important. Among all amino acids there are some essential amino acids and non-essential amino acids.
Those amino acids which cannot be prepared inside the body are known as essential amino acids. For example, leucine, lysine, histidine, etc. And those amino acids which can be produced in our body are called non-essential amino acids. For example, aspartic acid, glycine, proline, etc.
If any amino acids have an extra amine group then it is called basic amino acid and if any amino acid has an extra acidic group then it is called an acidic amino acid. The side chain (R group) is unique for every amino acid. Depending upon the side-chain R group amino acid can be hydrophilic or hydrophobic.
Among the given options serine has a hydroxyl methyl group in this side chain. The structure of serine is as follows,

So, the correct option is A.
Note: The isoelectric point of the amino acid is the pH at which a molecule carries no net electric charge or is electrically neutral in the statistical mean. Consider option A and B, at \[pH = 5.97\] glycine does not migrate while lysine moves to the cathode. At this pH, glycine is present in the form of zwitterion, as the pH is equal to the isoelectric point of glycine. At \[pH = 9.6\] , lysine does not migrate while glycine moves to the anode. At this pH, lysine is present in the form of zwitterion as the pH is equal to the isoelectric point of lysine.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




