
Which of the following are polyamide polymers?
A. Nylon-6,10
B. Nylon-6,6
C. Nylon-5
D. Perlon-U
Answer
594.6k+ views
Hint:
We know that polymer of polyamide is made up of monomer units in which amide group is present. Amide group is the $CO-N{{H}_{2}}$and the polyamide contains the $CO-NH$ linkage. Check all options whether there is amide group present or not.
Complete step by step solution:
The Nylon-6,10 is a polymer made up of the monomer units hexamethylenediamine and sebacoyl chloride. In this polymer, there is $CO-NH$ (amide) linkage and therefore, it is a polyamide polymer.
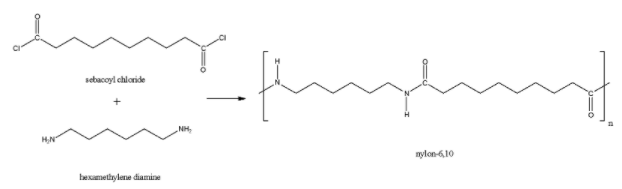
The Nylon-6,6 is a polymer made up of the monomer units hexamethylenediamine and adipic acid. Here also, the acid group and amine group joined together to form the amide linkage. So, Nylon-6,6 is also a polyamide polymer.
Nylon-5 is formed from the monomer units of pentamethylene diamine and sebacic acid. Here also, there is an amide linkage present. So, Nylon-5 is a polyamide polymer.
The perlon-U is also known as polyurethane also formed through the polyamide linkage after the combination of 1,4-butanediol and 1,6-hexamethylene diisocyanate. So, perlon-U is also a polyamide polymer.
Here, in each example, we can see that at least one $CO-NH$ bond.
Therefore, Nylon-6,10, Nylon-6,6, Nylon-5 and Perlon-U are polyamide polymers. So, the correct option is (a), (b), (c) and (d).
Note:
The name Nylon-6,10 suggests that one monomer unit of Nylon contains 6 carbons and the other monomer units contains 10 carbons. Similarly, Nylon-6,6 contains monomer units hexamethylenediamine and adipic acids, both having 6 carbon atoms.
We know that polymer of polyamide is made up of monomer units in which amide group is present. Amide group is the $CO-N{{H}_{2}}$and the polyamide contains the $CO-NH$ linkage. Check all options whether there is amide group present or not.
Complete step by step solution:
The Nylon-6,10 is a polymer made up of the monomer units hexamethylenediamine and sebacoyl chloride. In this polymer, there is $CO-NH$ (amide) linkage and therefore, it is a polyamide polymer.

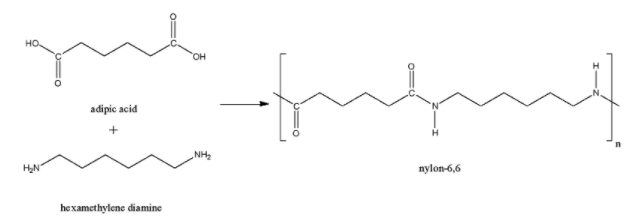
The Nylon-6,6 is a polymer made up of the monomer units hexamethylenediamine and adipic acid. Here also, the acid group and amine group joined together to form the amide linkage. So, Nylon-6,6 is also a polyamide polymer.

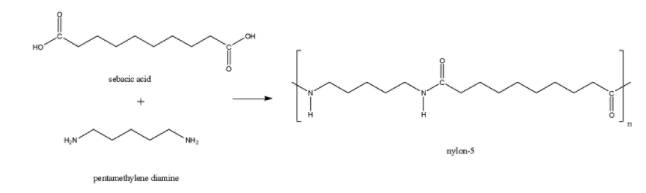
Nylon-5 is formed from the monomer units of pentamethylene diamine and sebacic acid. Here also, there is an amide linkage present. So, Nylon-5 is a polyamide polymer.

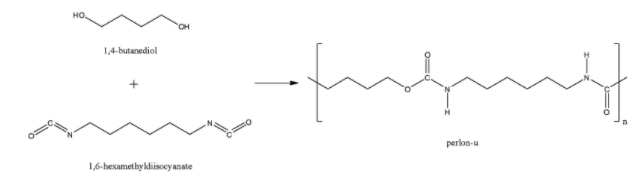
The perlon-U is also known as polyurethane also formed through the polyamide linkage after the combination of 1,4-butanediol and 1,6-hexamethylene diisocyanate. So, perlon-U is also a polyamide polymer.

Here, in each example, we can see that at least one $CO-NH$ bond.
Therefore, Nylon-6,10, Nylon-6,6, Nylon-5 and Perlon-U are polyamide polymers. So, the correct option is (a), (b), (c) and (d).
Note:
The name Nylon-6,10 suggests that one monomer unit of Nylon contains 6 carbons and the other monomer units contains 10 carbons. Similarly, Nylon-6,6 contains monomer units hexamethylenediamine and adipic acids, both having 6 carbon atoms.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




