
Which of the following compounds exhibits stereoisomerism?
A.2-methyl butene-1
B.3-methyl butyne-1
C.3- methyl butanoic acid
D.2- methyl butanoic acid
Answer
531.3k+ views
Hint: Before solving this question, we should know about stereoisomerism. The isomers have the same structural formula/ same parts but the arrangement of atoms and groups in the space is different. They are known as stereoisomers and this phenomenon is stereoisomerism. Now we can see which of the above options exhibits stereoisomerism.
Complete answer:
There are two kinds of stereoisomers that are enantiomers and diastereomers.
Enantiomers are compounds that are a mirror image of each other but are not superimposable. They do not have the plane of symmetry, so we cannot divide them into two halves but they are optically active.
Diastereomers are compounds that are not a mirror image of each other and have different physical and chemical properties.
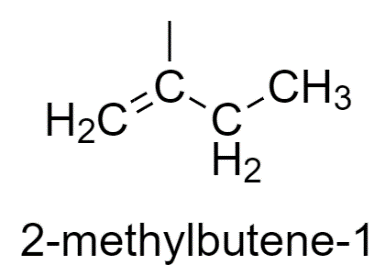
2-methyl butene-1 has two similar groups, therefore does not show stereoisomerism.
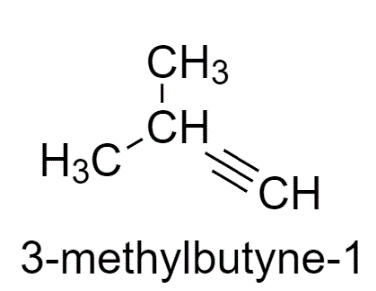
3-methyl butyne-1 has two similar groups, therefore does not show stereoisomerism.
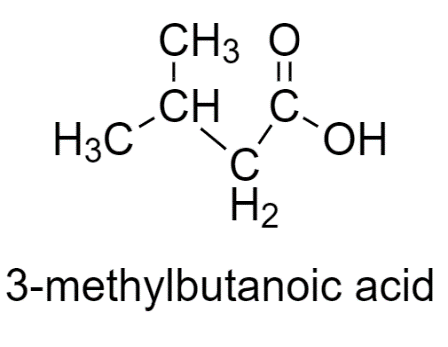
3-methylbutanoic acid has two similar groups, therefore does not show stereoisomerism.
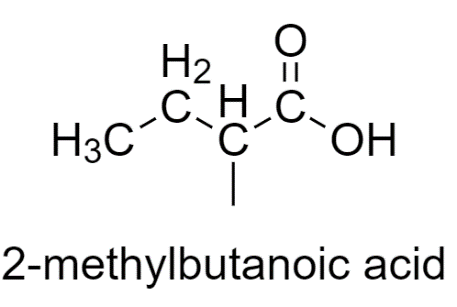
2-methylbutanoic acid has different substituents, so it shows stereoisomerism.
So Option (D) 2- methylbutanoic acid is correct.
Note:
Chirality and stereoisomers play a vital role in the field of chemistry as it tells us about the reason behind the formation of various organic structures. It contributes to the pharmacological activity of anesthetics. The ability to taste and smell is taken care of by the stereoisomers in our mouths.
Complete answer:
There are two kinds of stereoisomers that are enantiomers and diastereomers.
Enantiomers are compounds that are a mirror image of each other but are not superimposable. They do not have the plane of symmetry, so we cannot divide them into two halves but they are optically active.
Diastereomers are compounds that are not a mirror image of each other and have different physical and chemical properties.

2-methyl butene-1 has two similar groups, therefore does not show stereoisomerism.

3-methyl butyne-1 has two similar groups, therefore does not show stereoisomerism.

3-methylbutanoic acid has two similar groups, therefore does not show stereoisomerism.

2-methylbutanoic acid has different substituents, so it shows stereoisomerism.
So Option (D) 2- methylbutanoic acid is correct.
Note:
Chirality and stereoisomers play a vital role in the field of chemistry as it tells us about the reason behind the formation of various organic structures. It contributes to the pharmacological activity of anesthetics. The ability to taste and smell is taken care of by the stereoisomers in our mouths.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




