
Which of the following compounds undergo aldol condensation?
A. Formaldehyde
B. Trichloroacetaldehyde
C. Trimethyl acetaldehyde
D. Acetaldehyde
Answer
583.2k+ views
Hint: We know that, alpha $\left( \alpha \right)$ hydrogen is the hydrogen which is bonded to alpha carbon. Alpha carbon is the first carbon which is bonded to the functional group. Functional groups are the atoms or groups of atoms which decide the chemical reactivity of the compound, such as aldehyde, ketone, carboxylic acid etc.
Complete step by step answer: First we discuss the aldol condensation reaction. It is the reaction in which two aldehydes or ketones having alpha hydrogen undergo reaction in presence of dilute alkali to form $\beta $-hydroxy aldehyde or $\beta $-hydroxy ketone. So, it is clear that, to undergo aldol condensation, there must be alpha hydrogen $\left( \alpha \right)$ in the aldehyde or ketone.
Let’s find the correct answer from options. Option A is formaldehyde.
The structure of formaldehyde is,
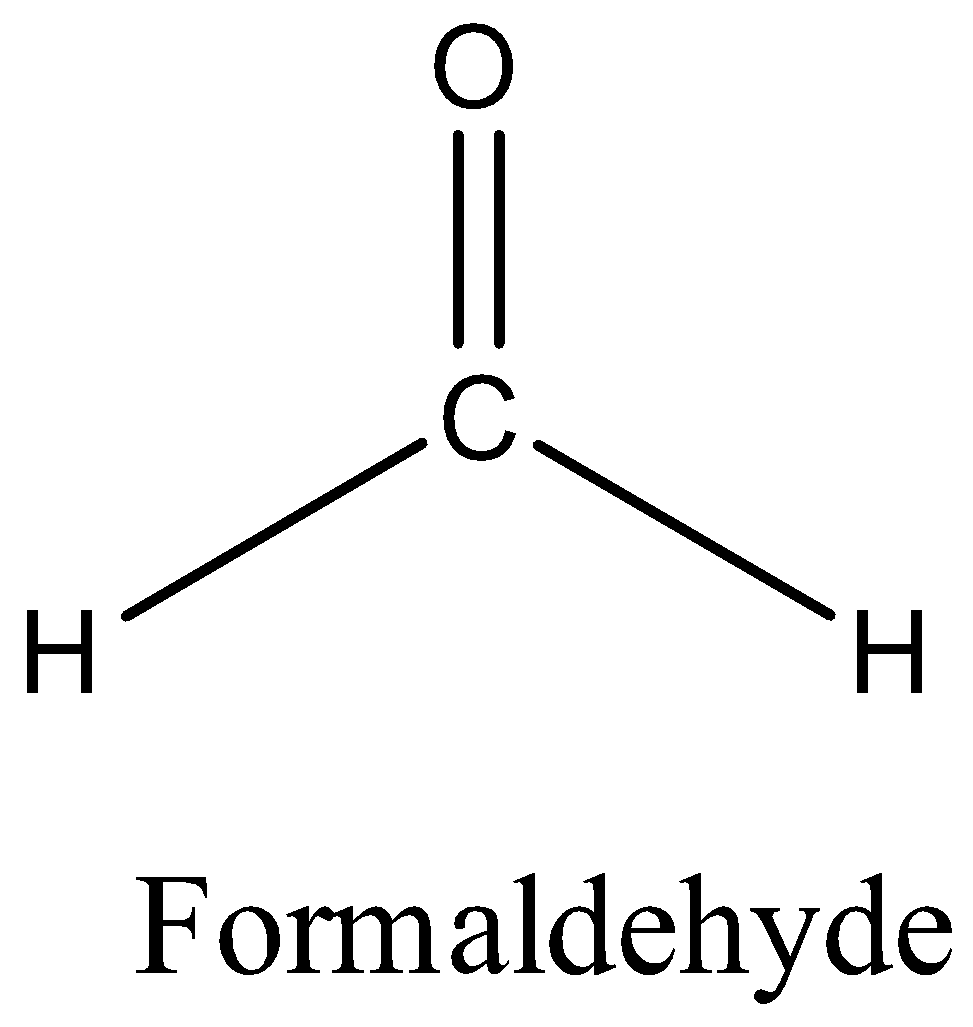
There is no alpha carbon in formaldehyde. So, there is no alpha hydrogen also. Therefore, formaldehyde cannot undergo aldol condensation.
Option B is Trichloroacetaldehyde. The structure of Trichloro acetaldehyde is,
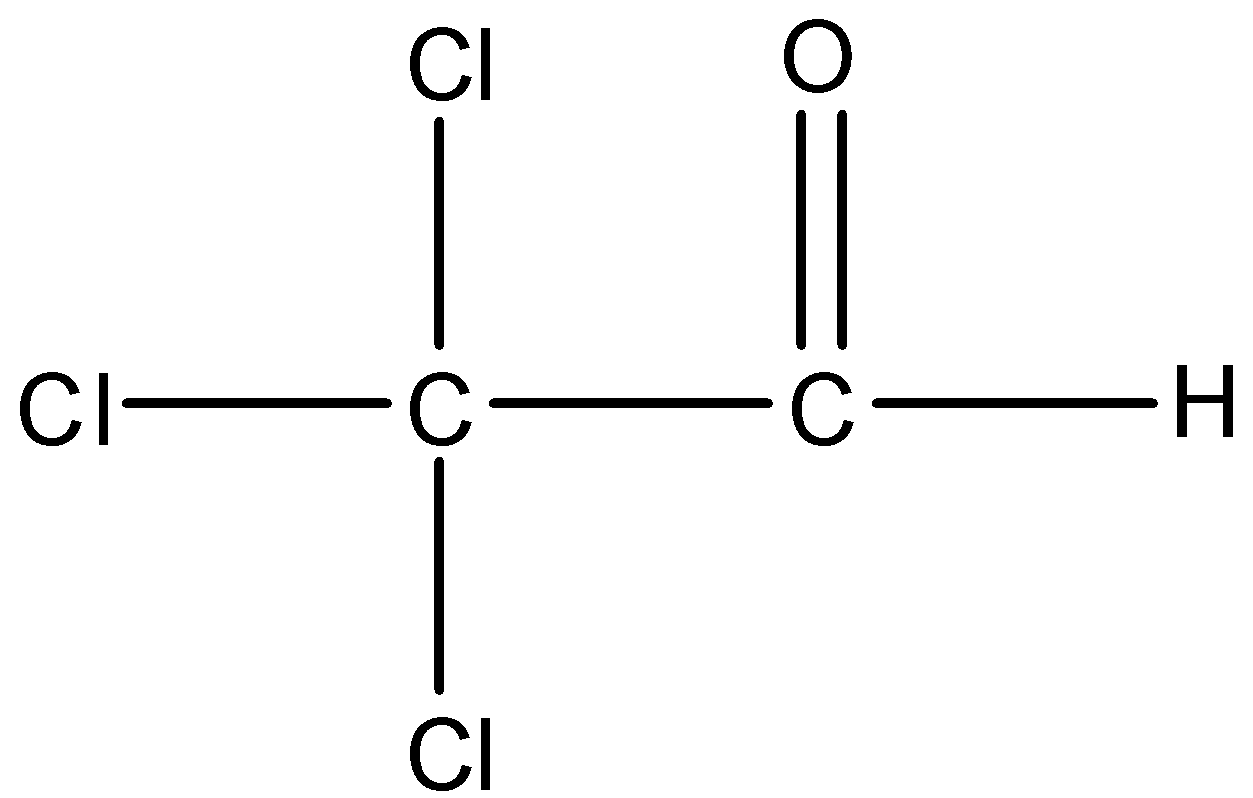
There is an alpha carbon but no alpha hydrogen is present in the compound. So, Trichloroacetaldehyde cannot undergo aldol condensation.
Option C is Trimethyl acetaldehyde. The structure of Trimethyl acetaldehyde is,
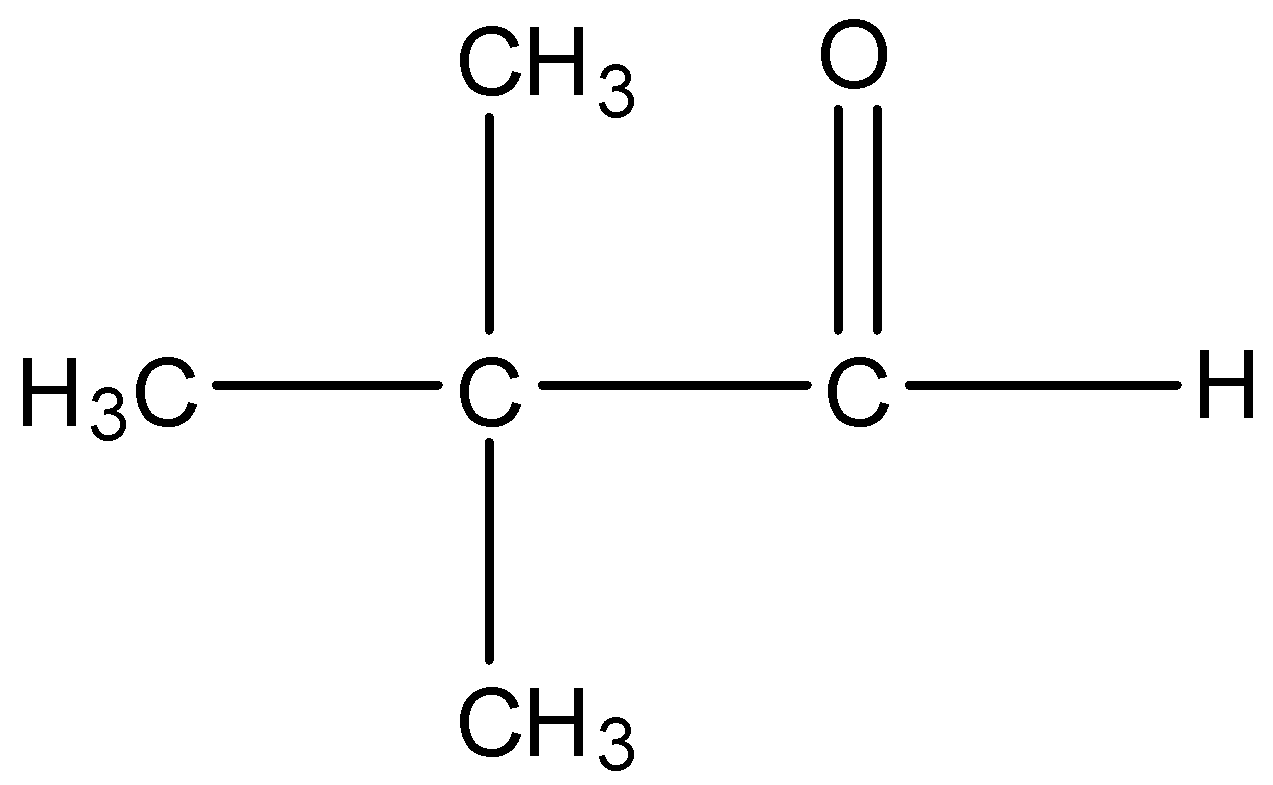
There is an alpha carbon but no alpha hydrogen is present in the compound. So, Trimethyl acetaldehyde cannot undergo aldol condensation.
Option D is acetaldehyde. The structure of acetaldehyde is,
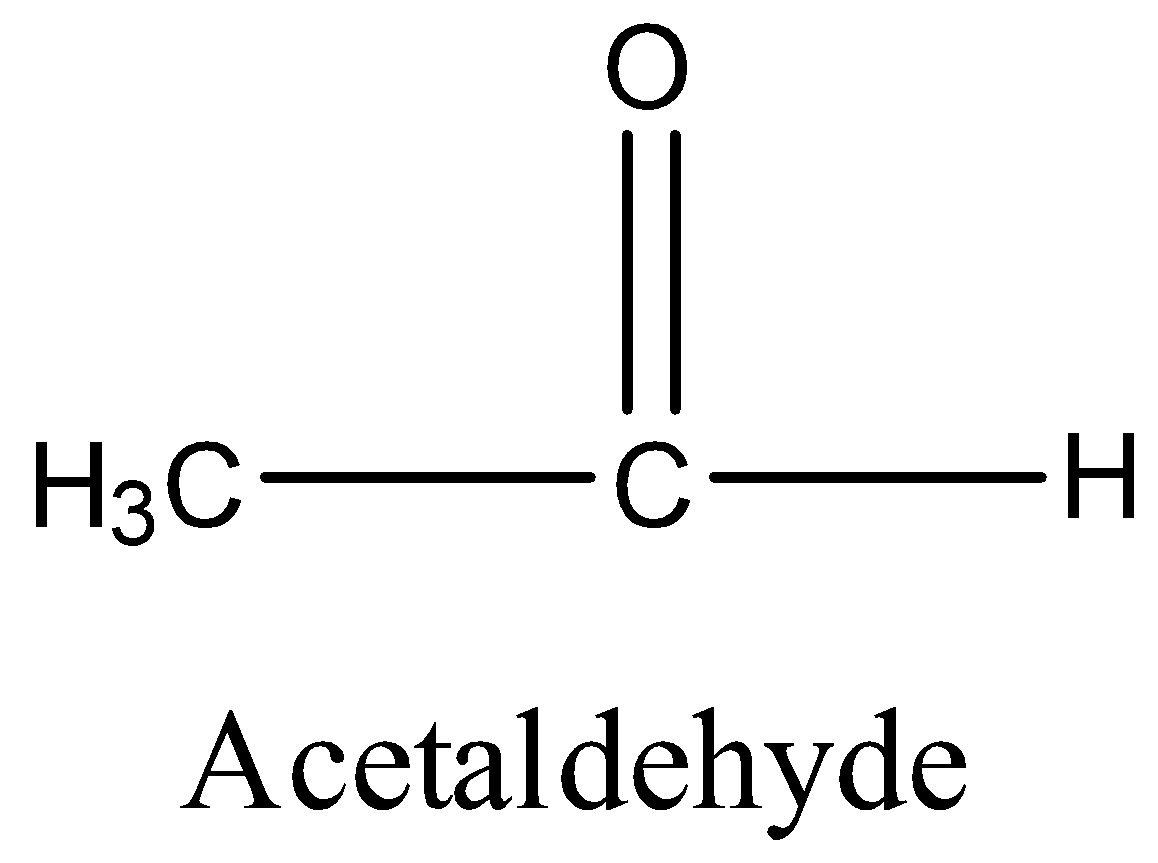
In acetaldehyde, alpha hydrogen is present. So, it undergoes aldol condensation
Hence, option D is the correct answer.
Note: On carrying aldol condensation between two different aldehydes or ketone, the reaction is called a cross aldol reaction. If both the reactants (aldehydes or ketones) have alpha hydrogen, a mixture of four products is produced.
Complete step by step answer: First we discuss the aldol condensation reaction. It is the reaction in which two aldehydes or ketones having alpha hydrogen undergo reaction in presence of dilute alkali to form $\beta $-hydroxy aldehyde or $\beta $-hydroxy ketone. So, it is clear that, to undergo aldol condensation, there must be alpha hydrogen $\left( \alpha \right)$ in the aldehyde or ketone.
Let’s find the correct answer from options. Option A is formaldehyde.
The structure of formaldehyde is,

There is no alpha carbon in formaldehyde. So, there is no alpha hydrogen also. Therefore, formaldehyde cannot undergo aldol condensation.
Option B is Trichloroacetaldehyde. The structure of Trichloro acetaldehyde is,

There is an alpha carbon but no alpha hydrogen is present in the compound. So, Trichloroacetaldehyde cannot undergo aldol condensation.
Option C is Trimethyl acetaldehyde. The structure of Trimethyl acetaldehyde is,

There is an alpha carbon but no alpha hydrogen is present in the compound. So, Trimethyl acetaldehyde cannot undergo aldol condensation.
Option D is acetaldehyde. The structure of acetaldehyde is,

In acetaldehyde, alpha hydrogen is present. So, it undergoes aldol condensation
Hence, option D is the correct answer.
Note: On carrying aldol condensation between two different aldehydes or ketone, the reaction is called a cross aldol reaction. If both the reactants (aldehydes or ketones) have alpha hydrogen, a mixture of four products is produced.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




