
Which of the following given compounds can exhibit tautomerism?
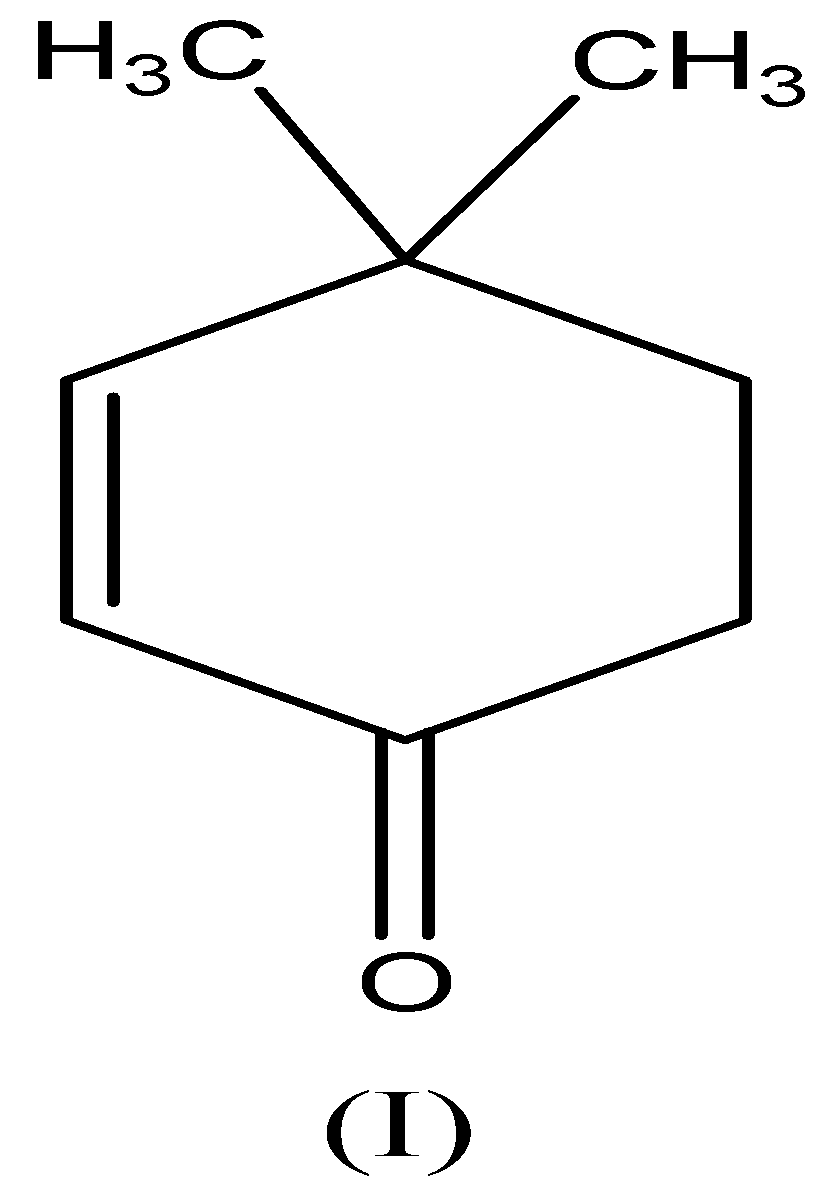
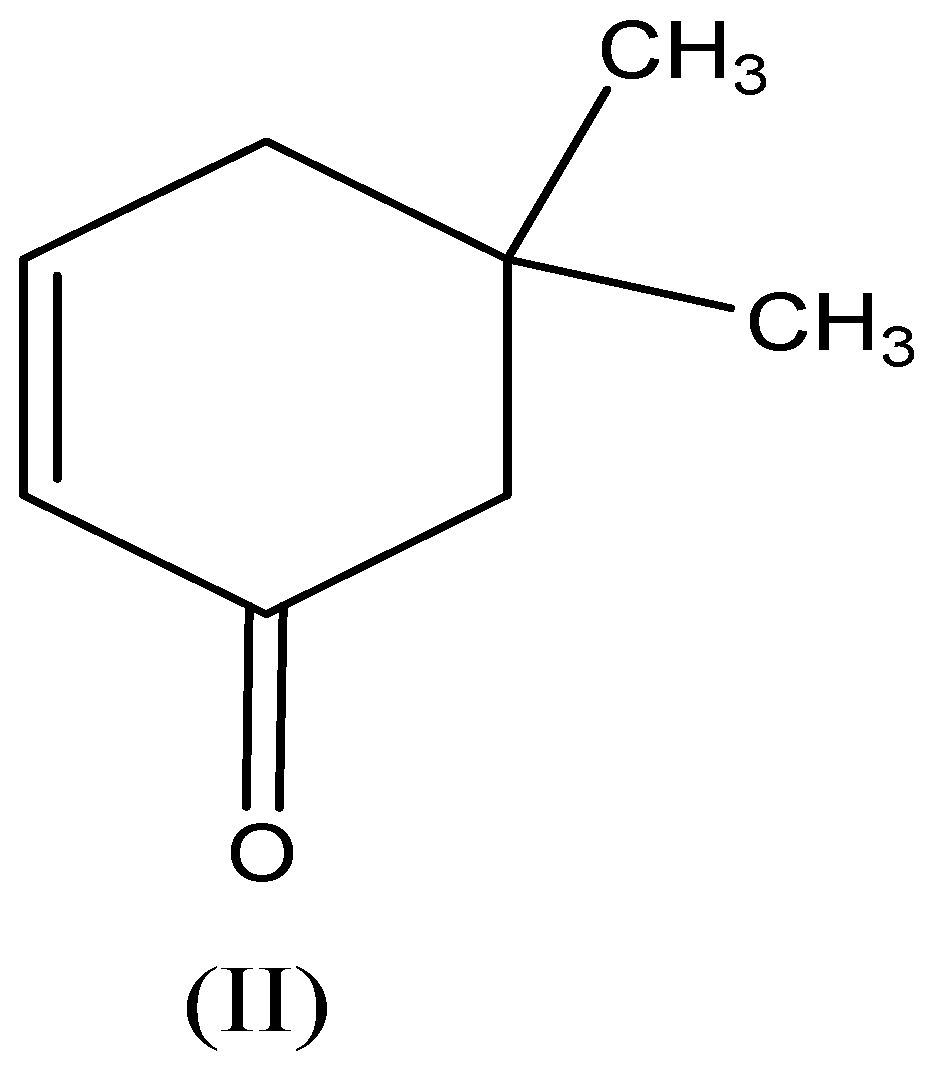
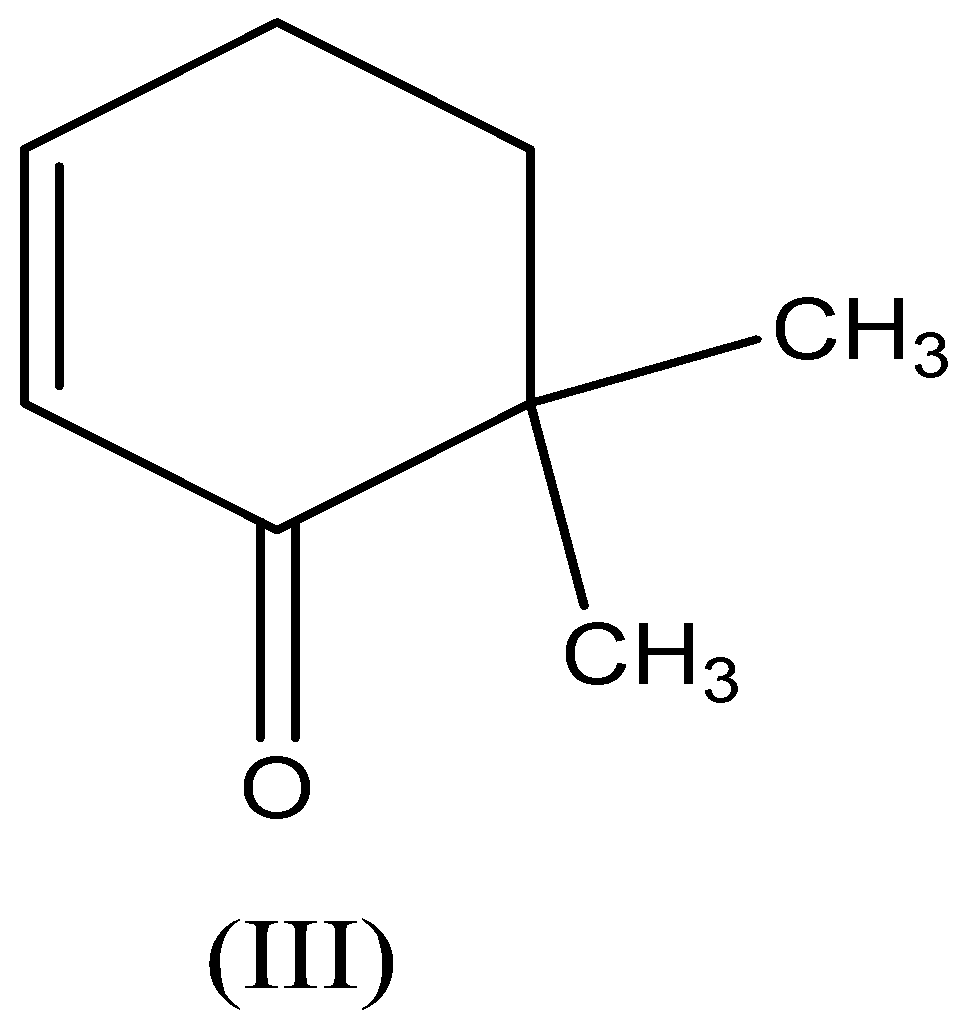
A. II and III
B. I,II and III
C. I and II
D. I and III



Answer
563.1k+ views
Hint: We have to remember that the different compounds with the same molecular formula are called isomers. Same molecular formula compounds behave as different compounds and this phenomenon is known as isomerism. Tautomerism is defined as two structural isomers, which differ in the relative positions of their atoms, and they are in rapid equilibrium.
Complete step by step answer:
We have to know that the keto-enol tautomerism is formed by the $\alpha - H$ in the keto form, this is in equilibrium, in fact it is rapidly interconvert to enol form, so, the $\alpha - H$ ; the bond between $\alpha - C$ and hydrogen moves over and forms a double bond. The pi bond of the carbonyl group, that breaks and the loosed proton is attracted by the oxygen atom and thus we get to enol form. Tautomerism other examples are, nitro-aci tautomerism, oxime-nitroso tautomerism and imine-enamine tautomerism.
Let us see the compounds one by one to which exhibit tautomerism.
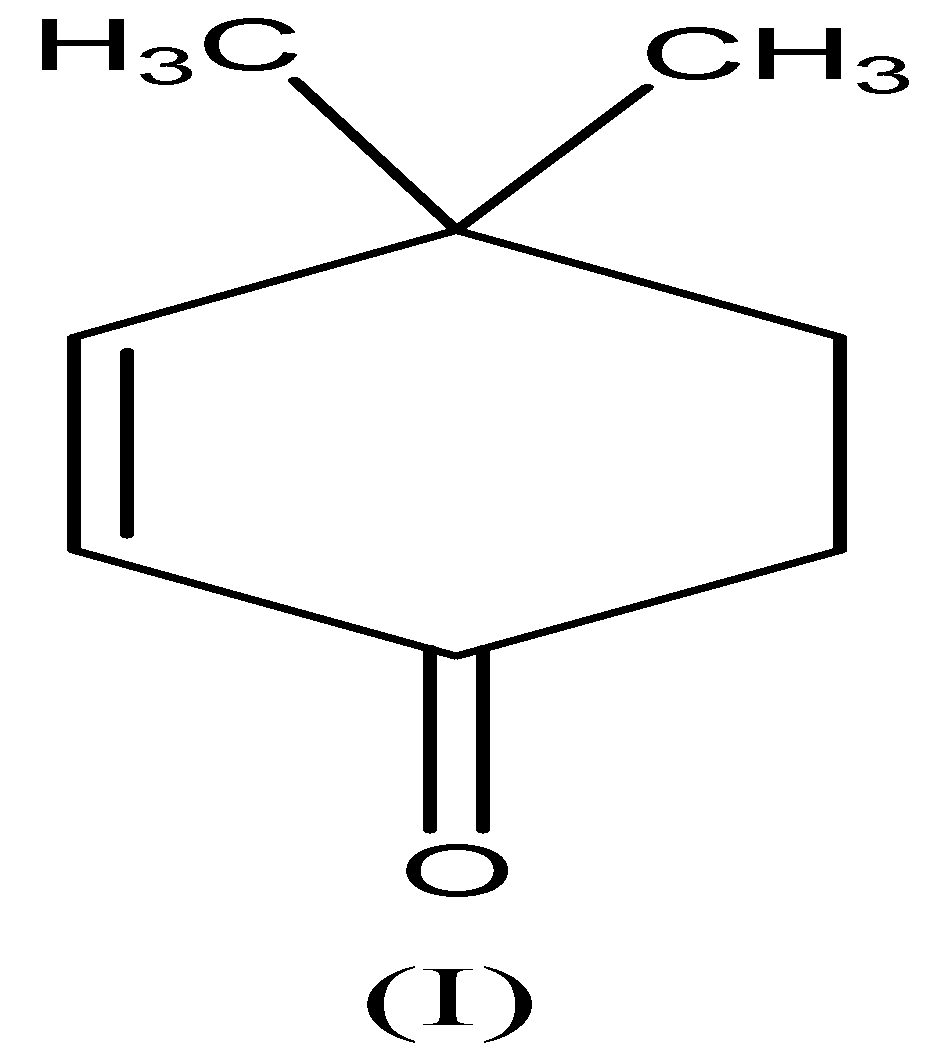
We need to know that the compound (I) is called \[{\text{4,4 - dimethylcyclohex - 2 - enone}}\] . This compound contains $\alpha - H$ and electron-withdrawing group and has the tendency to accepts the hydrogen atom, so this compound interconvert to enol form and exhibit keto-enol tautomerism as follows,
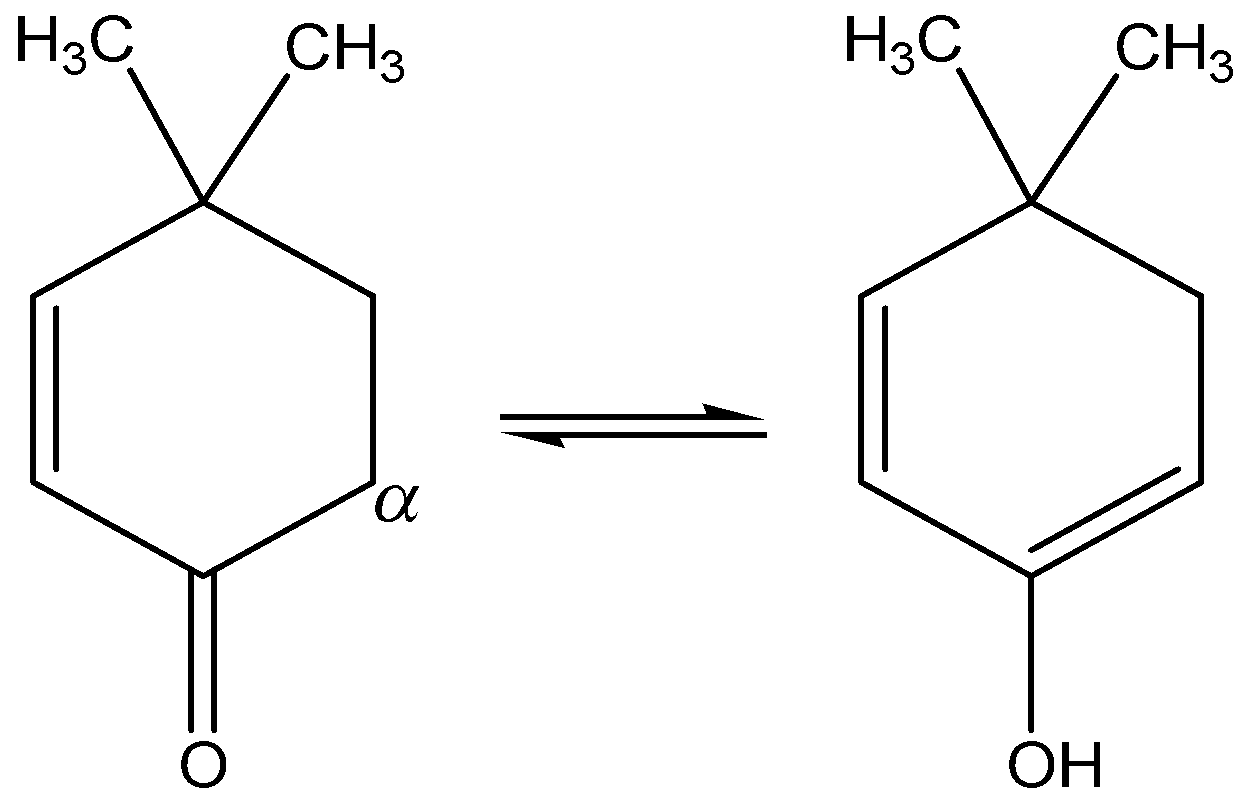
$Compound (I)$
We need to know that the compound (II) -$\text5,5 - dimethylcyclohex - 2 - enone$ also exhibits keto-enol tautomerism because of carbon contains an electron-withdrawing group, the tautomerism as follows,
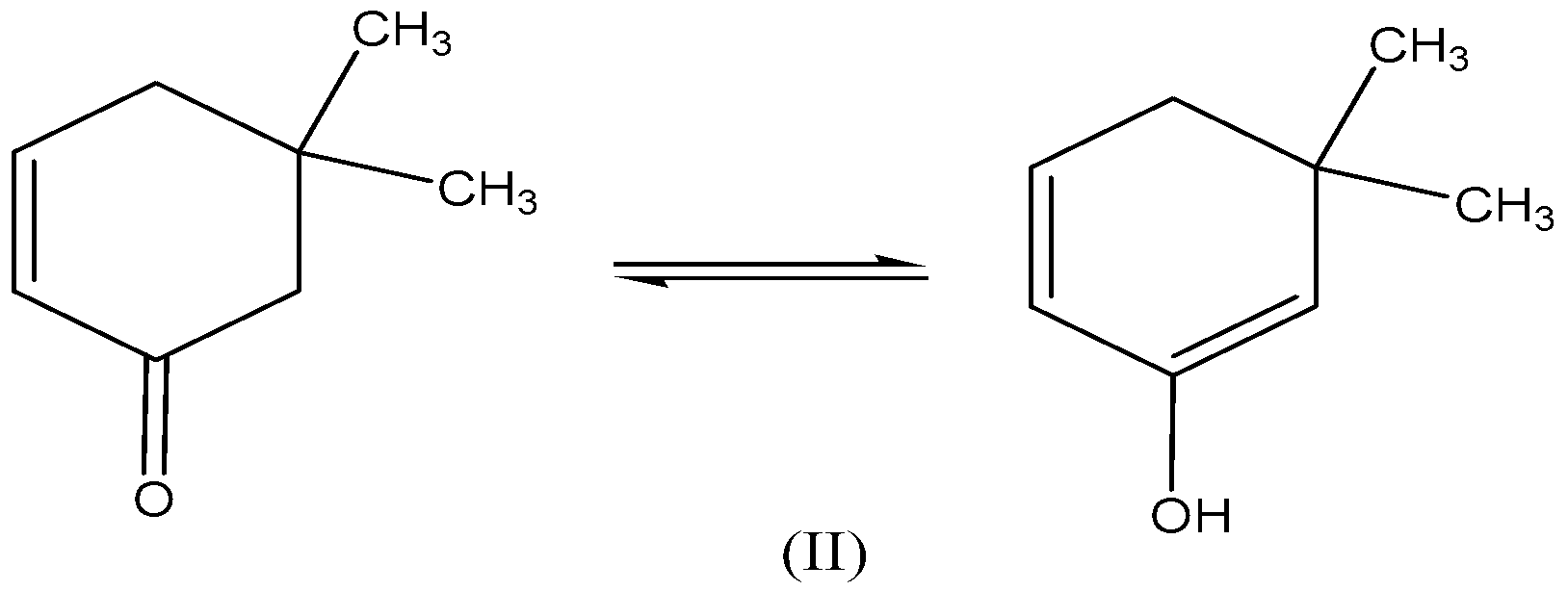
Compound (III)- \[{\text{6,6 - dimethylcyclohex - 2 - enone}}\] , in this, the more electronegative atom is attached to the carbon atom and has the tendency to accepts the hydrogen. Here $\gamma - H$ participates in tautomerism,
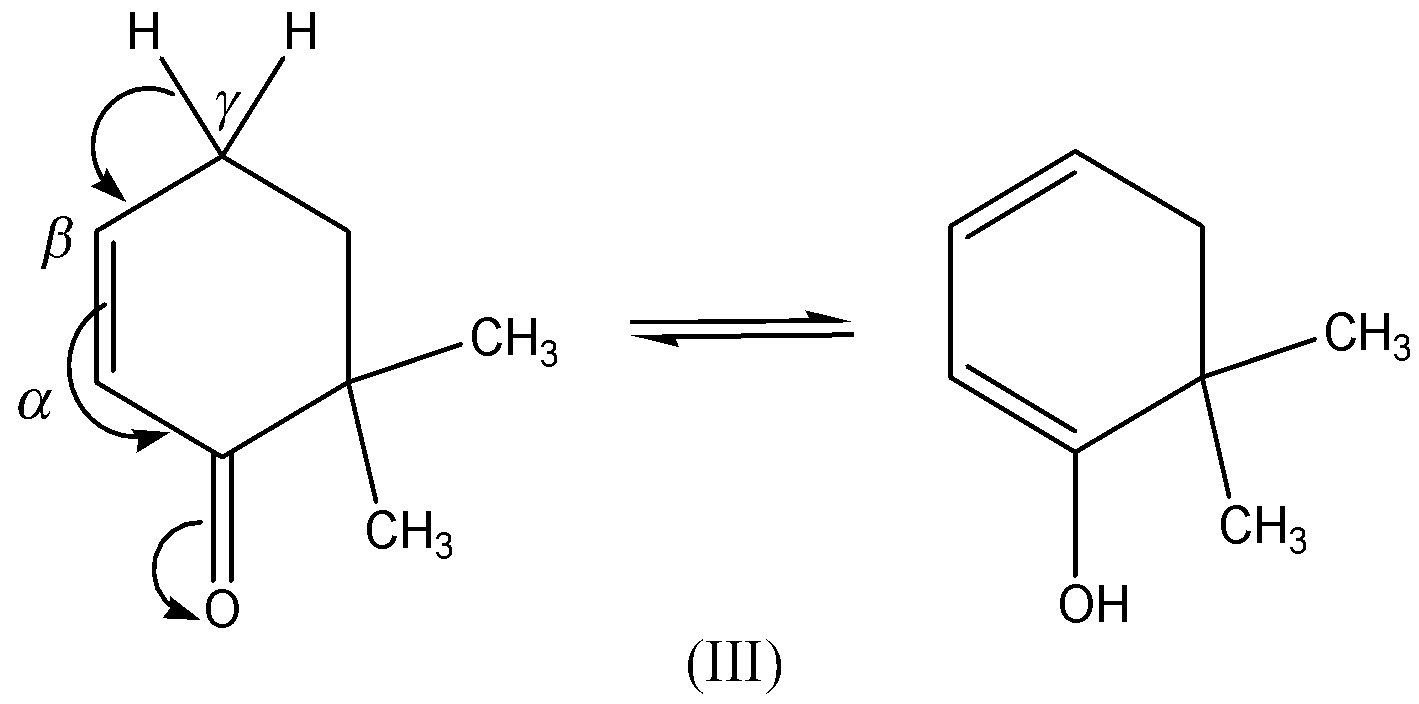
Hence the correct option is B.
Note: We need to know that the keto enol form of $C{H_2}C = OC{H_2}COO{C_2}{H_5}$ was successfully achieved by Knorr in $1911$ . We need to remember that the keto form lies more in equilibrium, the keto form is more stable than enol form in tautomerism, because the carbon-oxygen double bond is stronger than the carbon-carbon double bond. In contrast to resonance form keto-enol forms have no independence existence.
Complete step by step answer:
We have to know that the keto-enol tautomerism is formed by the $\alpha - H$ in the keto form, this is in equilibrium, in fact it is rapidly interconvert to enol form, so, the $\alpha - H$ ; the bond between $\alpha - C$ and hydrogen moves over and forms a double bond. The pi bond of the carbonyl group, that breaks and the loosed proton is attracted by the oxygen atom and thus we get to enol form. Tautomerism other examples are, nitro-aci tautomerism, oxime-nitroso tautomerism and imine-enamine tautomerism.
Let us see the compounds one by one to which exhibit tautomerism.

We need to know that the compound (I) is called \[{\text{4,4 - dimethylcyclohex - 2 - enone}}\] . This compound contains $\alpha - H$ and electron-withdrawing group and has the tendency to accepts the hydrogen atom, so this compound interconvert to enol form and exhibit keto-enol tautomerism as follows,

$Compound (I)$
We need to know that the compound (II) -$\text5,5 - dimethylcyclohex - 2 - enone$ also exhibits keto-enol tautomerism because of carbon contains an electron-withdrawing group, the tautomerism as follows,

Compound (III)- \[{\text{6,6 - dimethylcyclohex - 2 - enone}}\] , in this, the more electronegative atom is attached to the carbon atom and has the tendency to accepts the hydrogen. Here $\gamma - H$ participates in tautomerism,

Hence the correct option is B.
Note: We need to know that the keto enol form of $C{H_2}C = OC{H_2}COO{C_2}{H_5}$ was successfully achieved by Knorr in $1911$ . We need to remember that the keto form lies more in equilibrium, the keto form is more stable than enol form in tautomerism, because the carbon-oxygen double bond is stronger than the carbon-carbon double bond. In contrast to resonance form keto-enol forms have no independence existence.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




