
Which of the following gives haloform test positive
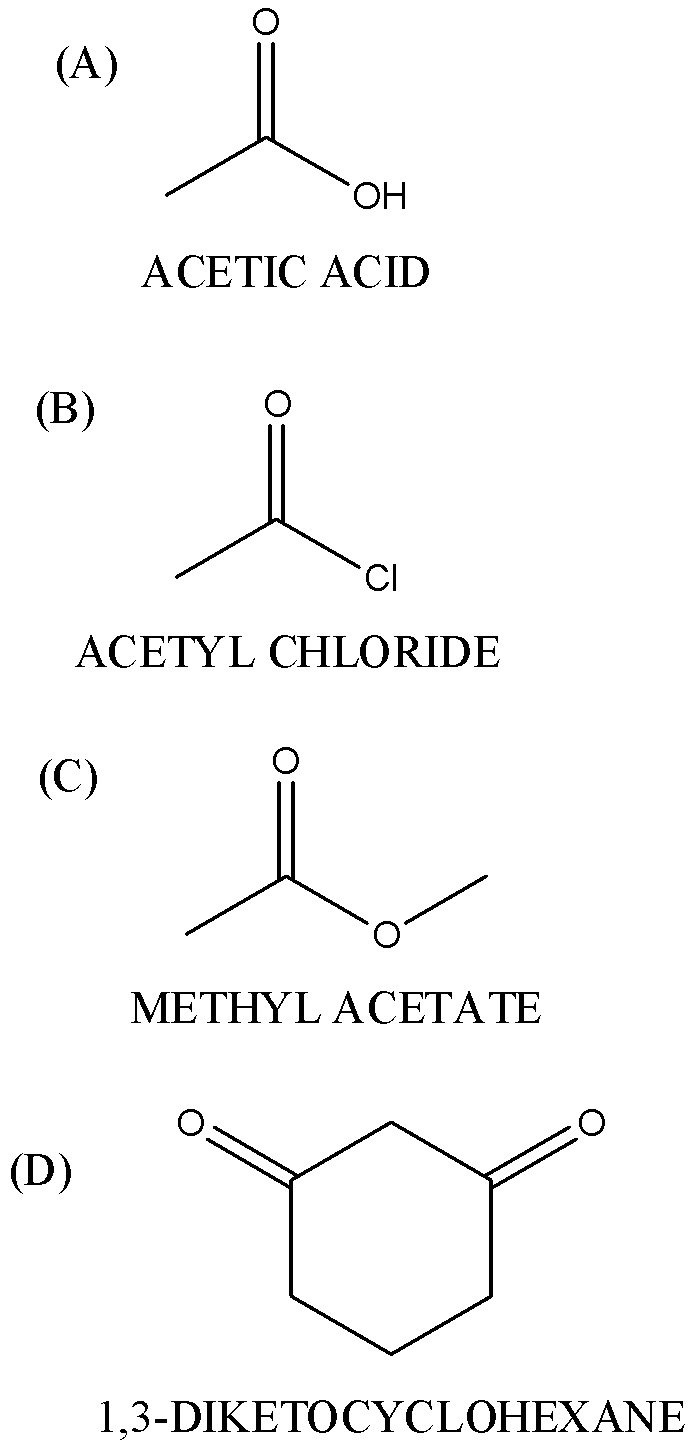

Answer
588.9k+ views
Hint: Aldehydes or ketones having at least methyl group attached to carbonyl carbon atom (methyl ketones) are oxidized by sodium hypohalite to sodium salts of corresponding carboxylic acid having one carbon atom less than that of carbonyl compound. Methyl group is converted to haloform. Aldehydes or ketones having methyl ketone groups give positive tests.
Complete solution step by step:
-Aldehydes or ketones having at least one methyl group linked to carbonyl carbon atom (methyl ketones) are oxidized by sodium hypohalite to sodium salts of corresponding carboxylic acids having one carbon atom less than that of carbonyl compound.
-Methyl group is converted to haloform.
\[RCOC{{H}_{3}}\xrightarrow{NaOH}RCOONa+CH{{X}_{3}}\]
-Carbon carbon double bond is not affected by oxidation.
-when aldehyde or ketone containing methyl keto group is treated with sodium hydroxide and iodine, sodium hydroxide with iodine forms sodium hypoiodite which oxidizes methyl keto group and yellow precipitate of iodoform is produced.
-Ethyl alcohol also gives positive haloform tests. As iodine is a mild oxidizing agent, Ethyl alcohol oxidizes to acetaldehyde. Acetaldehyde has a methyl ketone group. It gives a positive haloform test. Aldehyde is only aldehyde to give positive haloform test. Secondary alcohols with \[C{{H}_{3}}CH(OH)\]group also give haloform test as iodine or halogens are mild oxidizing agents which oxidizes it to methyl ketone groups.
-Carboxylic acid and ester do not give haloform test because due to resonance, the carbonyl group is not available.
(B)
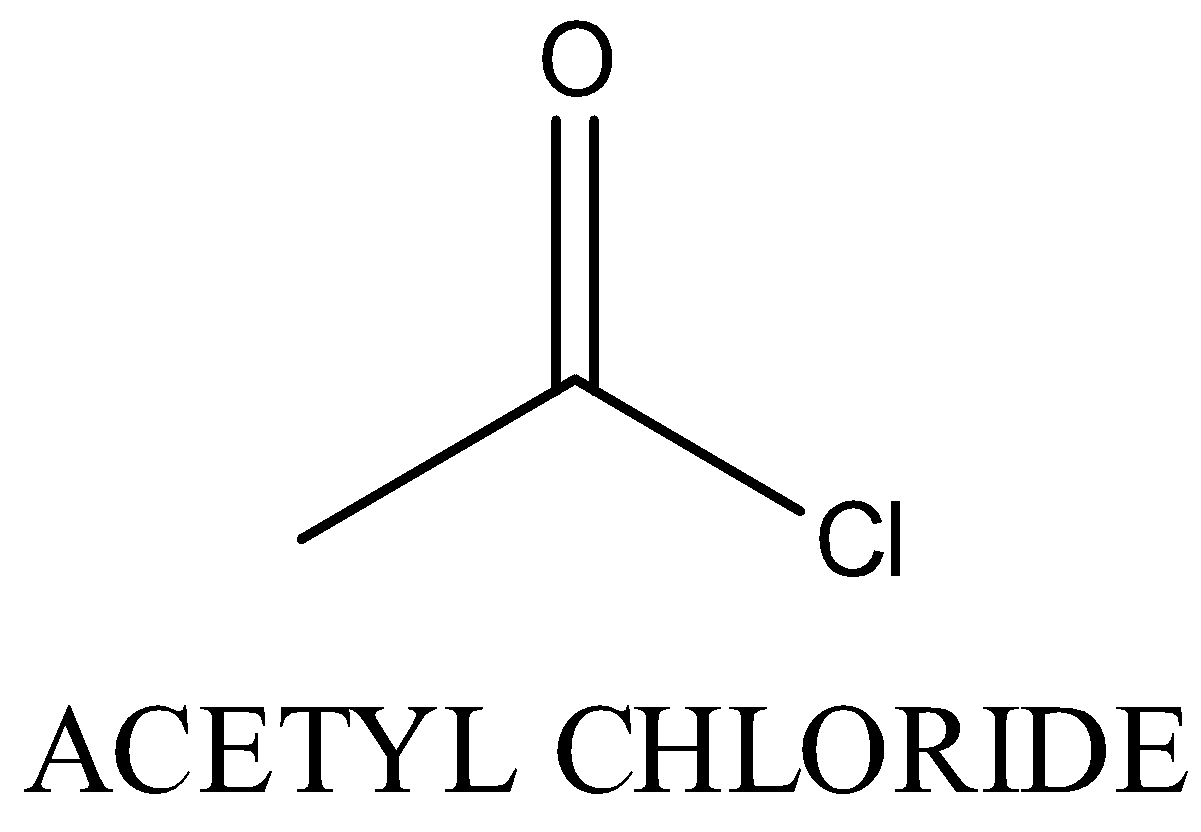 Gives positive haloform test
Gives positive haloform test
Note: Tertiary alcohols do not give haloform test as they do not undergo oxidation easily. Acetaldehyde is only aldehyde that gives positive haloform test. Ethyl alcohol is the only primary alcohol that gives positive haloform test. When methyl ketones are treated with sodium hydroxide and iodine, yellow precipitate of iodoform is produced which indicates positive haloform test. Sodium salt of carboxylic acid with one less carbon atom is also produced.
Complete solution step by step:
-Aldehydes or ketones having at least one methyl group linked to carbonyl carbon atom (methyl ketones) are oxidized by sodium hypohalite to sodium salts of corresponding carboxylic acids having one carbon atom less than that of carbonyl compound.
-Methyl group is converted to haloform.
\[RCOC{{H}_{3}}\xrightarrow{NaOH}RCOONa+CH{{X}_{3}}\]
-Carbon carbon double bond is not affected by oxidation.
-when aldehyde or ketone containing methyl keto group is treated with sodium hydroxide and iodine, sodium hydroxide with iodine forms sodium hypoiodite which oxidizes methyl keto group and yellow precipitate of iodoform is produced.
-Ethyl alcohol also gives positive haloform tests. As iodine is a mild oxidizing agent, Ethyl alcohol oxidizes to acetaldehyde. Acetaldehyde has a methyl ketone group. It gives a positive haloform test. Aldehyde is only aldehyde to give positive haloform test. Secondary alcohols with \[C{{H}_{3}}CH(OH)\]group also give haloform test as iodine or halogens are mild oxidizing agents which oxidizes it to methyl ketone groups.
-Carboxylic acid and ester do not give haloform test because due to resonance, the carbonyl group is not available.
(B)

Note: Tertiary alcohols do not give haloform test as they do not undergo oxidation easily. Acetaldehyde is only aldehyde that gives positive haloform test. Ethyl alcohol is the only primary alcohol that gives positive haloform test. When methyl ketones are treated with sodium hydroxide and iodine, yellow precipitate of iodoform is produced which indicates positive haloform test. Sodium salt of carboxylic acid with one less carbon atom is also produced.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




