
Which of the following has the weakest carbon- chloride bond?
A.
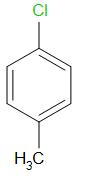
B.
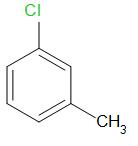
C.
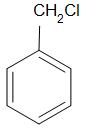
D.
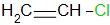



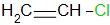
Answer
573.3k+ views
Hint:Bond energy is termed as the amount of energy that is required to break the bonds in a mole of molecules. More is the bond energy stronger is the bond. To answer this question, we must study the molecules and determine which one of the following will have stronger bonds between the carbon atom and the chlorine atom.
Complete step by step solution:
We know that, in chlorobenzene, the chlorine atom is directly bonded to the benzene ring and thus, the carbon chloride bond is very stable. Thus, the initial structure of chlorobenzene is more stable as compared to benzyl chloride. Moreover, due to the presence of lone pairs on the chlorine atom, it undergoes resonance with benzene ring. The delocalization of lone pair electrons provides extra stability and there is a partial double bond character in the carbon- chlorine bond. Thus, the carbon chlorine bond is very strong and requires high energy to break.
Thus, we can rule out options A and B
Now we have benzyl chloride, which is an allylic chloride, and a vinyl chloride. The carbon atom in vinyl chloride is $s{p^2}$ hybridized whereas that in benzyl chloride is $s{p^3}$ hybridized. The carbon atom in the vinyl chloride is more electronegative than the carbon present in the benzyl chloride. Thus, the carbon chlorine bond is stronger in the vinyl chloride.
Thus, the correct answer is C.
Note:
Alkyl chlorides and phenyl chlorides are very different in terms of their bonding. The carbon chloride bond in alkyl chlorides is very weak as compared to the carbon chloride bonds in
Complete step by step solution:
We know that, in chlorobenzene, the chlorine atom is directly bonded to the benzene ring and thus, the carbon chloride bond is very stable. Thus, the initial structure of chlorobenzene is more stable as compared to benzyl chloride. Moreover, due to the presence of lone pairs on the chlorine atom, it undergoes resonance with benzene ring. The delocalization of lone pair electrons provides extra stability and there is a partial double bond character in the carbon- chlorine bond. Thus, the carbon chlorine bond is very strong and requires high energy to break.
Thus, we can rule out options A and B
Now we have benzyl chloride, which is an allylic chloride, and a vinyl chloride. The carbon atom in vinyl chloride is $s{p^2}$ hybridized whereas that in benzyl chloride is $s{p^3}$ hybridized. The carbon atom in the vinyl chloride is more electronegative than the carbon present in the benzyl chloride. Thus, the carbon chlorine bond is stronger in the vinyl chloride.
Thus, the correct answer is C.
Note:
Alkyl chlorides and phenyl chlorides are very different in terms of their bonding. The carbon chloride bond in alkyl chlorides is very weak as compared to the carbon chloride bonds in
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




